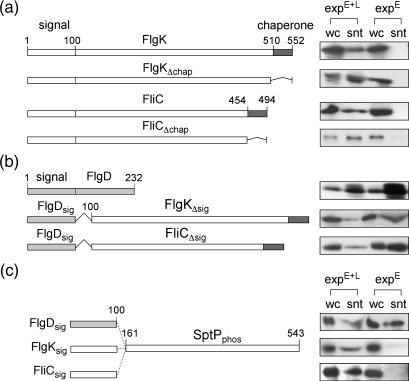
Fig. 4.
Influence of subunit domains on sorting. (a) Export of chaperoned late subunits (FlgK and FliC) and their variants that cannot bind chaperone (FlgKΔchap and FliCΔchap) in export pathways that are either early locked (expE; ΔflgEKL27 ΔflgE, SJW1353 acquired from Ohnishi et al.28) or native (expE + L; ΔflgKL or ΔfliC). Proteins from whole cells (wc) and supernatants (snt) were immunoblotted with FlgK or FliC antisera. (b) Export of FlgD and recombinant late subunits FlgKΔsig and FliCΔsig lacking amino acids 1–100 fused to residues 1–100 of early FlgD in export pathways (described above) that are either early locked (expE) or native (expE + L). Proteins from whole cells (wc) and supernatants (snt) were immunoblotted with FlgD, FlgK or FliC antisera. (c) Export of recombinant fusion proteins comprising putative early or late subunit N-terminal signal regions (FlgDsig, FlgKsig and FliCsig; amino acids 1–100) fused to the signal-less SptP tyrosine phosphatase domain (SptPphos; residues 161–543) in early locked (expE; ΔflgE) and native (expE + L; ΔfliC) pathways. Proteins were immunoblotted with SptP antisera (V. Koronakis, University of Cambridge). Genes encoding variant wild-type and variant FliC, FlgK, FlgD and SptP were amplified by overlap extension PCR using Salmonella chromosomal DNA as template. PCR products were inserted into XbaI–HindIII restriction sites of the pBAD18 expression vector. Recombinant genes were expressed (LB, 0.01% arabinose) and proteins were assayed as in Fig. 2b. Control experiments performed in isogenic ΔfliI and ΔflgEfliI strains (created by P22 transduction of ΔfliI allele into ΔflgE) showed that none of the recombinant proteins was exported (data not shown).