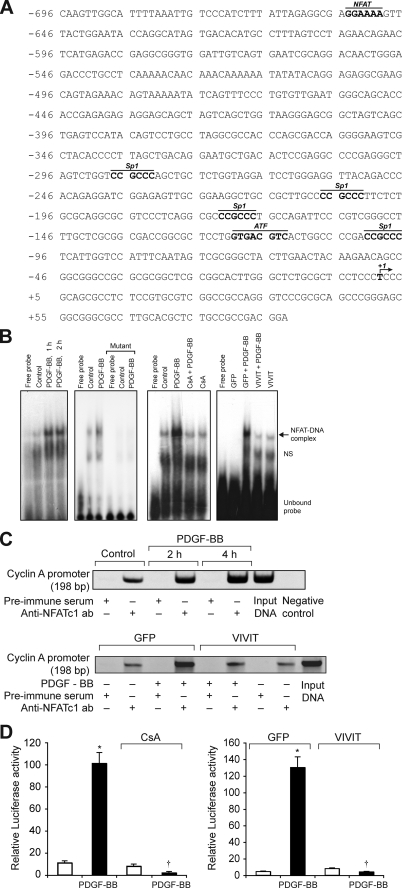
FIGURE 4.
PDGF-BB induces cyclin A promoter-driven luciferase reporter gene activity in NFAT-dependent manner in VSMC. A, rat cyclin A promoter region showing the potential transcription factor-binding motifs. B, 1st panel from left, quiescent VSMC were treated with and without PDGF-BB (20 ng/ml) for the indicated times, and nuclear extracts were prepared. An equal amount of nuclear protein from control and each treatment was analyzed for DNA binding activity using 32P-labeled NFAT consensus sequences from rat cyclin A promoter as a probe. 2nd panel, 32P-labeled consensus or mutant NFAT probes in the DNA binding assays using control and 1 h-PDGF-BB (20 ng/ml)-treated nuclear extracts. 3rd panel, all the conditions were the same as in 1st panel except that cells were treated with and without PDGF-BB (20 ng/ml) in the presence and absence of CsA (10 μm) for 1 h, and nuclear extracts were prepared and analyzed for NFAT DNA binding activity. 4th panel, conditions were the same as in 3rd panel except that cells were transduced with either Ad-GFP or Ad-VIVIT at an m.o.i. of 80 and quiesced before subjecting to treatment with PDGF-BB, preparation of nuclear extracts, and measuring NFAT DNA binding activity. C, top panel, chromatin immunoprecipitation assay was performed with control and various time periods of PDGF-BB-treated VSMC using monoclonal anti-NFATc1 antibodies, and the resulting DNA fragments were subjected to PCR amplification using primers spanning the NFAT consensus sequences from rat cyclin A promoter. Bottom panel, VSMC were transduced with either Ad-GFP or Ad-VIVIT at an m.o.i. of 80, quiesced, treated with and without PDGF-BB (20 ng/ml) for 2 h, and subjected to ChIP assay as described in the top panel. D, left panel, VSMC that were transfected with cyclin A promoter-luciferase construct and quiesced were treated with and without PDGF-BB (20 ng/ml) in the presence and absence of CsA (10 μm) for 8 h, and cell extracts were prepared and assayed for luciferase activity. Right panel, all the conditions were the same as in the left panel except that cells were transduced first with Ad-GFP or Ad-VIVIT at an m.o.i. of 80 and then transfected with luciferase reporter plasmid DNA followed by quiescence and treatment with and without PDGF-BB and measuring luciferase activity. *, p < 0.01 versus control or GFP; †, p < 0.01 versus PDGF-BB or GFP + PDGF-BB treatment alone.