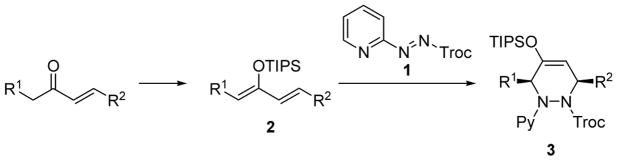
The hetero-Diels-Alder reaction is one of the most useful reactions in organic chemistry because multi functionalized compounds can be constructed in a single step.1 The catalytic enantioselective version of this process has attracted much attention in modern organic chemistry. We recently reported the catalytic highly enantioselective nitroso hetero-Diels-Alder reaction using nitroso-pyridine as a dienophile in the presence of a chiral copper catalyst.2 Encouraged by this success, we focused on hetero-Diels-Alder reaction using a 2-azopyridine derivative since this reaction with azo compounds (azo hetero-Diels-Alder reaction) produces 1,4-diamines.3 These structural motifs are important building blocks as well as 1,4-amino alcohols. For example, these structures are found in pharmaceutically important compounds such as HIV protease inhibitors.4 Diastereoselective azo hetero-Diels-Alder reactions using a chiral auxiliary have been developed,5 however, despite several efforts toward an enantioselective version of this process,6 there are no reports of a catalytic highly enantioselective azo hetero-Diels-Alder reaction. We herein report the catalytic highly regio- and enantioselective azo hetero-Diels-Alder reaction (Scheme 1).
Scheme 1.
Azo hetero-Diels-Alder Reaction
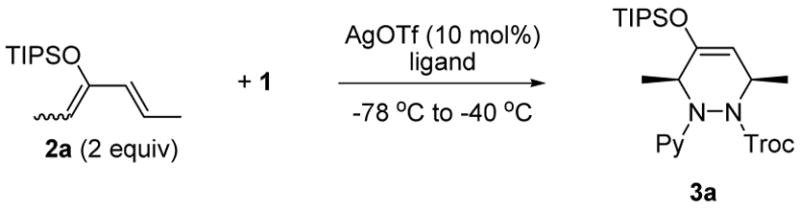
2-Azopyridine (1) was prepared in two steps from commercially available 2-hydrazinopyridine.7 On the basis of our previous results, we chose for initial investigations the hetero- Diels-Alder reaction of acyclic silyloxydiene 2a with (R)-BINAP and CuPF6(CH3CN)4 catalyst.2, 8 Unfortunately, we were unable to observe any chiral induction. Thus, several metal catalysts were surveyed9 and we found that the combination of AgOTf and (R)-BINAP in THF produced adduct 3a with 55% ee. Encouraged by this result, various ligands and solvents were tested (Table 1). The use of (R)-BINAP as a ligand and CH3CN or EtCN as a solvent gave 3a with 94% ee (Table 1, entries 5 and 6). EtCN was selected as a solvent to obtain high reproducibility. Next, the ratio of (R)-BINAP and AgOTf was checked since we previously had observed that three types of Ag-BINAP complex were formed in THF.10 The 2:1 ratio of AgOTf and (R)-BINAP was found to be optimal, producing an adduct 3a with >99% ee. It should be noted that decreased enantioselectivity was observed by chiral biphosphine ligands with narrow dihedral angles (entries 7 and 8) which are expected to generate a 1:1 complex of Ag-ligand preferentially.
Table 1.
Optimization of Reaction Conditions
 | ||||
|---|---|---|---|---|
| entry | ligand | solvent | yield (%) | ee (%)b |
| 1 | (R)-BINAP (10 mmol%) | THF | 73 | 55 |
| 2 | (R)-BINAP (10 mol%) | Et2O | 74 | 56 |
| 3 | (R)-BINAP (10 mol%) | toluene | 63 | 67 |
| 4 | (R)-BINAP (10 mol%) | CH2Cl2 | 72 | 80 |
| 5a | (R)-BINAP (10 mol%) | CH3CN | 61 | 94 |
| 6 | (R)-BINAP (10 mol%) | EtCN | 62 | 94 |
| 7 | (R)-Difluorophos (10 mol%) | EtCN | 76 | 30 |
| 8 | (R)-Segphos (10 mol%) | EtCN | 71 | 20 |
| 9 | (R)-BINAP (5 mol%) | EtCN | 87 | >99 |
| 10 | (R)-BINAP (20 mol%) | EtCN | 26 | 0 |
Reaction was conducted at −40 °C.
ee value was determined by HPLC (Supporting Information).
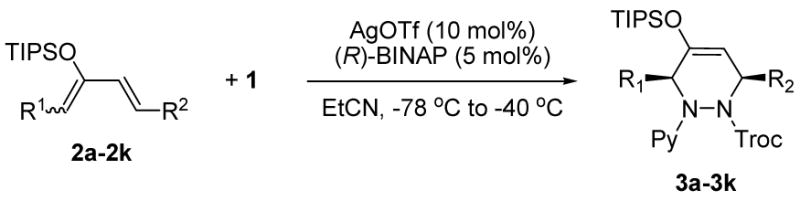
Having an optimized condition in hand, the applicability of this reaction was studied for the functionalized silyloxydienes 2b–2j.11 All of the reactions proceeded in high yields and enantio-selectivities, with complete regio- and diastereoselectivities.
The dialkyl-substituted dienes generally gave high enantio-selectivites (Table 2, entries 1, 2 and 5). Silyloxydiene 2c with a sterically hindered substituent afforded 3c with slightly decreased enantioselectivity. Lewis basic substituents such as ester, ether, protected alcohols, and protected amine (Table 2, entries 4 and 6–8) were also used in the reaction and produced highly functionalized products enantioselectively. Silyloxydiene 2j having 2-furyl group gave an adduct 3j with high regio- and enantioselectivity (Table 2, entry 10). Meanwhile, the enantio-selectivity of reaction using silyloxydiene 2k with phenyl group was decreased dramatically (Table 2, entry 11).
Table 2.
Reaction with Various Dienesa
 | |||||
|---|---|---|---|---|---|
| entry | diene | R1 | R2 | yield (%) | ee (%)b |
| 1 | 2a | Me | Me | 87 | >99 |
| 2 | 2b | Me | n-C5H11 | 84 | 95 |
| 3 | 2c | Me | i-Pr | 65 | 84 |
| 4 | 2d | Me | CH2CH2CH2CO2Me | 74 | 98 |
| 5 | 2e | Bn | Me | 74 | 92 |
| 6 | 2f | 4-MOMO-Bn | Me | 85 | 90 |
| 7 | 2g | CH2CH2CH2OTBS | i-Bu | 82 | 95 |
| 8 | 2h | CH2CH2CH2OTBS | CH2OBn | 84 | 98 |
| 9 | 2i | CH2CH2CH2NNsBoc | i-Bu | 77 | 98 |
| 10 | 2j | Me | 2-Furyl | 78 | 92 |
| 11c | 2k | Me | Ph | 70 | 55 |
Reaction was conducted with AgOTf (10 mol%), (R)-BINAP (5 mol%), azopyridine (1 equiv), and silyloxydiene (2 equiv) under Ar at −78 °C and gradually warmed to −40 °C over 3 h.
ee value was determined by HPLC (Supporting Information).
20 mol% of AgOTf and 10 mol% (R)-BINAP were used.
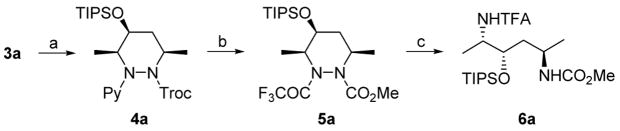
The products can be cleanly converted into the corresponding diamino alcohols. For example, deprotection of TIPS group of 3a with TBAF/AcOH12 followed by reduction and protection of the resulting alcohol gave 4a as a single diastereomer. Removal of the pyridine ring was cleanly achieved by the known procedure,2c) accompanied by the conversion of 2,2,2-trichloroethoxycarbonyl group to methoxycarbonyl group. The resulting amine was protected with trifluoroacetyl group to afford 5a. To cleave N-N bond of 5a, 5a was treated with SmI2 to give 6a in 71% yield (Scheme 2).13 Thus, two amino groups are differentiated for further transformation.
Scheme 2. Conversion to Protected Diamino Alcohola.
a(a) (i) TBAF, AcOH, (ii) NaBH4, (iii) TIPSOTf, NEt3, 65% (3 steps); (b) (i) MeOTf (ii) NaOH, (iii) TFAA, NEt3, 71% (3 steps); (c) SmI2, MeOH, 71%.
The absolute and relative configurations of azo hetero-Diels-Alder adducts were assigned by X-ray crystallographic analysis. Deprotection of Troc and TIPS groups followed by reduction afforded 7a as a single diastereomer. Subsequently, 7a was converted into 4-bromobenzoate derivative 8a which was crystallized from Et2O (Scheme 3, Supporting information).
Scheme 3. Determination of Absolute Stereochemistrya.
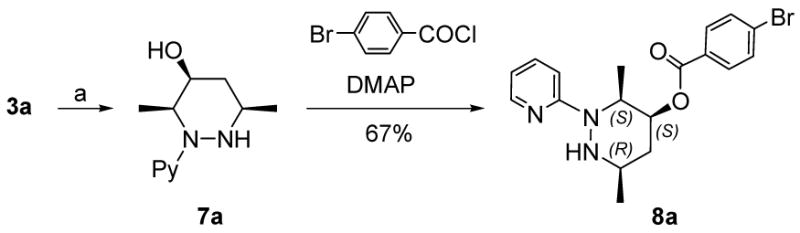
a(a) (i) Zn, AcOH, (ii) TBAF, AcOH, (iii) NaBH4, 53% (3 steps).
In summary, we have developed highly regio-, diastereo-, and enantioselective azo hetero-Diels-Alder reaction using 2-azo-pyridine (1) and silver(I)-BINAP 2:1-catalyst. This catalytic process could be one of the effective synthetic routes to a number of chiral 1,4-diamines which are pharmaceutically important compounds. Further studies of the detailed mechanism of the reaction and synthetic applications are currently underway in our laboratory.
Supplementary Material
Experimental details, spectroscopic data, including determination of absolute configuration, and complete ref. 4h. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
Support of this research was provided by National Institutes of Health (NIH) Grant No. GM068433-01 and Merck Research Laboratories. We thank Dr. Ian M. Steel for X-ray crystallographic analysis.
References
- 1.For a general review of hetero-Diels-Alder reactions, see: Fringuelli F, Taticchi A, editors. The Diels-Alder Reaction: Selected Practical Methods. John Wiley & Sons; 2002. Kobayashi S, Jørgensen KA, editors. Cycloaddition Reactions in Organic Synthesis. Wiley-VCH; Weinheim: 2002. pp. 15–09.Jacobsen EN, Pfaltz A, Yamamoto H, editors. Comprehensive Asymmetric Catalysis III. Springer; Berlin: 1999. pp. 123–254.Gouverneur V, Reiter M. Chem Eur J. 2005;11:5806. doi: 10.1002/chem.200500406.Jørgensen KA. Angew Chem Int Ed. 2000;39:3558.Tietze LF, Kettschau G. Top Curr Chem. 1997;189:1.
- 2.(a) Yamamoto Y, Yamamoto H. Eur J Org Chem. 2006:2031. [Google Scholar]; (b) Yamamoto Y, Yamamoto H. Angew Chem Int Ed. 2005;44:7082. doi: 10.1002/anie.200501345. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2005;117:7244. [Google Scholar]; (c) Yamamoto Y, Yamamoto H. J Am Chem Soc. 2004;126:4128. doi: 10.1021/ja049849w. [DOI] [PubMed] [Google Scholar]
- 3.(a) Chowdari NS, Ahmad M, Albertshofer K, Tanaka F, Barbas CF., III Org Lett. 2006;8:2839. doi: 10.1021/ol060980d. [DOI] [PubMed] [Google Scholar]; (b) Reetz MT. Chem Rev. 1999;99:1121. doi: 10.1021/cr980417b. [DOI] [PubMed] [Google Scholar]; (c) Patel M, Kaltenbach RF, III, Nugiel DA, McHugh RJ, Jr, Jadhav PK, Bacheler LT, Cordova BC, Klabe RM, Erickson -VS, Garber S, Reid C, Seitz SP. Bioorg Med Chem Lett. 1998;8:1077. doi: 10.1016/s0960-894x(98)00175-9. [DOI] [PubMed] [Google Scholar]; (d) Reedijk J. Chem Commun. 1996:801. [Google Scholar]; (e) Konradi AW, Pedersen SF. J Org Chem. 1992;57:28. [Google Scholar]; (f) Cummins CC, Beachy MD, Schrock RR, Vale MG, Sankaran V, Cohen RE. Chem Mater. 1991;3:1153. [Google Scholar]
- 4.(a) Izawa K, Onishi T. Chem Rev. 2006;106:2811. doi: 10.1021/cr050997u. [DOI] [PubMed] [Google Scholar]; (b) Specker E, Böttcher J, Brass S, Heine A, Lilie H, Schoop A, Müller G, Griebenow N, Klebe G. Chem Med Chem. 2006;1:106. doi: 10.1002/cmdc.200500008. [DOI] [PubMed] [Google Scholar]; (c) Engstrom K, Henry R, Hollis LS, Kotecki B, Marsden I, Pu Y-M, Wagaw S, Wang W. J Org Chem. 2006;71:5369. doi: 10.1021/jo060737s. [DOI] [PubMed] [Google Scholar]; (d) Yeung CM, Klein LL, Flentge CA, Randolph JT, Zhao C, Sun M, Dekhtyar T, Stoll VS, Kempf DJ. Bioorg Med Chem Lett. 2005;15:2275. doi: 10.1016/j.bmcl.2005.03.008. [DOI] [PubMed] [Google Scholar]; (e) Ikunaka M. Chem Eur J. 2003;9:378. doi: 10.1002/chem.200390039. [DOI] [PubMed] [Google Scholar]; (f) Abdel RHM, Al KGS, El KNA, Youssef AF, Kiso Y. Curr Med Chem. 2002;9:1905. doi: 10.2174/0929867023368890. [DOI] [PubMed] [Google Scholar]; (g) Ghosh AK, Bilcer G, Schiltz G. Synthesis. 2001:2203. doi: 10.1055/s-2001-18434. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Kempf DJ, et al. J Med Chem. 1998;41:602. doi: 10.1021/jm970636+. [DOI] [PubMed] [Google Scholar]; (i) Ettmayer P, Hübner M, Gstach H. Tetrahedron Lett. 1994;35:3901. [Google Scholar]; (j) Kempf DJ, Codacovi L, Wang XC, Kohlbrenner WE, Wideburg NE, Saldivar A, Vasavanonda S, Marsh KC, Bryant P, Sham HL, Green BE, Betebenner DA, Erickson J, Norbeck DW. J Med Chem. 1993;36:320. doi: 10.1021/jm00055a003. [DOI] [PubMed] [Google Scholar]
- 5.(a) Robiette R, Cheboub BK, Peeters D, Marchand BJ. J Org Chem. 2003;68:9809. doi: 10.1021/jo0302049. [DOI] [PubMed] [Google Scholar]; (b) Adam W, Bosio SG, Degen H-G, Krebs O, Stalke D, Schumacher D. Eur J Org Chem. 2002:3944. [Google Scholar]; (c) Urabe H, Kusaka K, Suzuki D, Sato F. Tetrahedron Lett. 2002;43:285. [Google Scholar]; (d) Zhang A, Kan Y, Jiang B. Tetrahedron. 2001;57:2305. [Google Scholar]; (e) Kaname M, Arakawa Y, Yoshifuji S. Tetrahedron Lett. 2001;42:2713. [Google Scholar]; (f) Dussault PH, Han Q, Sloss DG, Symonsbergen DJ. Tetrahedron. 1999;55:11437. [Google Scholar]; (g) Wyatt PB, Villalonga BC, Motevalli M. Tetrahedron Lett. 1999;40:149. [Google Scholar]; (h) Aspinall IH, Cowley PM, Mitchell G, Raynor CM, Stoodley RJ. J Chem Soc Perkin Trans 1. 1999:2591. [Google Scholar]; (i) Virgili M, Moyano A, Pericas MA, Riera A. Tetrahedron. 1999;55:3959. [Google Scholar]; (j) Aspinall IH, Cowley PM, Mitchell G, Stoodley RJ. J Chem Soc, Chem Commun. 1993:1179. [Google Scholar]
- 6.Aburel PS, Zhuang W, Hazell RG, Jørgensen KA. Org Biomol Chem. 2005;3:2344. doi: 10.1039/b503744a. [DOI] [PubMed] [Google Scholar]
- 7.Synthesis of similar compounds, see: Srinivasan V, Jebaratnam DJ, Budil DE. J Org Chem. 1999;64:5644. doi: 10.1021/jo990750v.
- 8.The reaction of 2-triisopropylsilyloxy-1,3-cyclohexadiene with CuPF6(CH3CN)4 and (R)-BINAP gave the corresponding adduct with >99% ee. The origin of this result is being investigated in our laboratory.
- 9.Reactions using the following metal catalysts were checked on TLC; Al, B, Mg, Zn, Ti, Sc, Hf, Yb, Zr, In, La. None of them accelerated the reaction.
- 10.Momiyama N, Yamamoto H. J Am Chem Soc. 2004;126:5360. doi: 10.1021/ja039103i. [DOI] [PubMed] [Google Scholar]
- 11.Synthesis of silyloxydienes, see: Nakashima D, Yamamoto H. J Am Chem Soc. 2006;128:9626. doi: 10.1021/ja062508t.Yamamoto Y, Yamamoto H. Angew Chem Int Ed. 2005;44:7082. doi: 10.1002/anie.200501345.
- 12.Excess amount of AcOH was necessary to avoid epimerization of methyl group via retro-Michael reaction.
- 13.SmI2 mediated cleavage of N-N bond with trifluoroacetyl group, see: Chowdari NS, Barbas CF., III Org Lett. 2005;7:867. doi: 10.1021/ol047368b.Ding H, Friestad GK. Org Lett. 2004;6:637. doi: 10.1021/ol036480r.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental details, spectroscopic data, including determination of absolute configuration, and complete ref. 4h. This material is available free of charge via the Internet at http://pubs.acs.org.