Abstract
Gastrointestinal stromal tumors (GISTs) are characterized by alterations in genes involved in cell cycle regulation. Although p16 (INK4A) have been extensively investigated in GISTs, there are still discrepancies regarding its prognostic value. Therefore, we evaluated the clinical occurrence, diagnostic and prognostic value of p16 staining in GIST. One hundred one patients (54 women and 47 men) with a mean age of 64.1 years (range, 17–94 years) were surgically treated for a GIST within a 10-year period. Of these patients, 28 (28%) were affected by metastases (mean follow-up, 4.5 years). In 36 patients (36%), GIST occurred coincidentally with other malignancies. Expression of c-kit was confirmed in 97 GIST patients (96%). In patients with high-risk GIST, the expression of p16 expression was highly predictive for poor prognosis, i.e., the development of recurrence or metastases (P = .006) and poor survival (P = .004). In addition, the expression of p16 was highly predictive for reduction of the survival in patients who were affected by metastases or recurrence (P = .041). The disease-specific and disease-free 1-, 3-, and 5-year survival rate was 96%, 90%, and 85% and 81%, 77%, and 72%, respectively. Primary tumor state, tumor size, and high-risk classification were confirmed as relevant predictors for unfavorable prognosis in GIST (P < .001). Our results indicate that in high-risk GIST and in patients with recurrence or metastases, the expression of p16 is highly predictive for poor outcome. Thus, in addition to high-risk classification, p16 expression might be an indicator for “very high risk GIST.”
Introduction
Gastrointestinal stromal tumors (GISTs) constitute the largest group of mesenchymal tumors with an overall incidence of 1 to 2 per 100,000 per year [1] and are characterized by the expression of CD117 (c-kit) [2–4]. Gain-of-function mutations of the c-kit gene resulting in consecutive changes in mitotic rate, proliferation, and differentiation are considered to be the major step in the pathogenesis of GIST [2,4–8]. Recently, a general risk classification was established (Table 1) [3,6,7]. However, regardless of several predicting parameters, among them tumor size and mitotic rate, the clinical behavior of GIST markedly varies [6]. Therefore, analysis of additional factors allowing a more detailed prognosis for the individual patient is still a pertinent issue.
Table 1.
Risk of Malignancy According to Fletcher et al.
| Risk | Size (cm) | Mitotic Activity per 50 HPFs |
| Very low risk | <2 | <5 |
| Low risk | 2–5 | <5 |
| Intermediate risk | <5 | 6–10 |
| 5–10 | <5 | |
| High risk | >5 | >5 |
| >10 | Any rate | |
| Any size | >10 |
Detailed characterization of several cell cycle regulators, particularly p16, revealed that they have a great impact on the pathogenesis of GIST. Loss of p16 has been reported to predict poor clinical outcome in a variety of several human tumors [9–14]. The prognostic significance of p16 gene alterations in GIST is still under debate [14–21]. Loss of p16 protein expression in GIST is described as a significant predictive value in some—but not all—studies. In contrast, an adverse effect of p16 protein expression on prognosis was recently described [22]. High-risk classification is a significant predictor of the probability of developing metastases or recurrence [3,7]. However, the subgroup of high-risk GIST is very heterogeneous. Unfortunately, at present, no significant predictors exist which divide high-risk GIST or which assess the prognosis of patients with GIST who experienced tumor recurrence or metastases.
In this study, we evaluated the clinicopathologic features, diagnostic appearance, and clinical outcome of patients with GISTs. Attention was focused on primary tumor state, on risk classification, and on estimation of the clinical outcome. Due to the conflicting data surrounding the prognostic value of p16 expression in GIST, the immunostaining pattern of p16 was additionally analyzed. Particular attention was directed to patients with high-risk GIST. An individual subgroup was created to analyze the prognostic value of p16 expression in this cohort.
Materials and Methods
Patients
Extending for 10 years, 101 GIST patients (54 women and 47 men) with a mean age of 64.1 years (range, 17–94 years) underwent surgical resection through laparotomy for a GIST in our department. After obtaining informed consent, chart review was carried out focusing on the following parameters: initial clinical symptoms, pathologic features, and clinical follow-up.
Clinical data were retrospectively reviewed based on the hospital records including medical history and on results from the contributing radiologists and pathologists. Medical history and radiographic data were collected if available in medical records. All 101 tumors were defined as GISTs based on the combination of histologic evaluation (highly cellular spindle/epithelioid/mixed cell tumors) and CD117 (c-kit) or—if negative—platelet-derived growth factor receptor alpha (PDGFR-α) positivity followed by prospective assessment of the clinical outcome.
Histologic Evaluation
Original hematoxylin and eosin-stained sections were reviewed in each case. Mitoses were counted from 50 consecutive high-power fields (HPFs) from the most cellular and mitotically active areas (area of an individual field 0.2 mm2). In all cases, the feature of cell type was recorded (spindle vs epithelioid or mixed cell-type). For the purpose of clinicopathologic comparison, GISTs were classified according to Fletcher et al. [3] (see also Table 1) and additionally divided in two groups: “Non-high-risk” including all patients classified as “Very low,” “Low,” or “Intermediate,” respectively and “High-risk.” In one patient with neoadjuvant Gleevec therapy, mitotic count was done on the initial cutting needle biopsy.
Immunohistochemical staining for CD117, PDGFR-α, and p16 was performed using the alkaline-phosphatase method in a Dako AutoStainer (Dako, Denmark). Representative paraffin-embedded tissue blocks were cut into 3-µm sections. After deparaffinization and antigen demasking through microwave heating (20 minutes, citrate buffer pH 6), the primary antibodies (p16INK4, 1:400; ZYTOMED Systems GmbH, Berlin, Germany), CD117 (c-kit polyclonal antibodies, at 1:200; Dako), and PDGFR-α (polyclonal antibodies, at 1:100; Dianova, Hamburg, Germany) were incubated on the slices followed by incubation with the alkaline phosphatase-conjugated streptavidin and the chromogenic substrate neofuchsin (Dako REAL Detection System). Counterstaining was done with hematoxylin.
According to Schneider-Stock et al. [14], p16 immunohistochemical staining was evaluated by estimating 10 HPFs. Nuclei of tumor cells with or without cytoplasmatic staining were counted according to a 4-point semiquantitative scale [no staining, 0–10% (0); weak, 11–20% (1); moderate, 21–50% (2); strong, >50% (3)]. A cutoff at 20% positivity in at least 10 HPF was used for prognostic analysis. Nontumorous stromal cells showing nuclear reactivity served as an internal control. In addition to the evaluation protocol of Schneider-Stock et al. [14], we also analyzed cutoff values at 10% and 50% and compared these results.
Statistical Analysis
For statistical analysis, the two-sided χ2 test or Fisher exact test in cross tables was used to demonstrate the relation between different qualitative outcome parameters, such as GIST-related death (yes/no), the occurrence of tumor recurrence or metastases (yes/no), and several independent variables with particular focus on p16 staining. For the analysis of the disease-specific survival (DSS) and disease-free survival (DFS), the total survival curves (Kaplan-Meier) were compared using the log-rank test. Deaths from unrelated causes were censored [14]. The study followed an explorative data analysis. Therefore, the P values were not adjusted for multiple testing and no single main outcome parameter was defined, all parameters were of equally interest. A P value of <.05 was considered statistically relevant. Calculations were performed using WinSTAT (R. Fitch Software, Germany), SAS/STAT (SAS Institute Inc., Cary, NC) and SPSS (SPSS Inc., Chicago, IL).
Results
One hundred one patients with a mean age of 64.1 years (SD, 13.4) and a male/female ratio of 54:47 (54% were men, 46% were women) underwent surgical resection for a GIST through laparotomy. Clinical manifestations, diagnostic imaging, pathologic findings, treatment options, and clinical outcome were evaluated.
Clinical Presentation
Clinical symptoms predominantly manifested as abdominal pain 36% (36/101), GI bleeding 28% (28/101), and unspecific symptoms such as fatigue and weight loss. However, 31 patients (31%) were asymptomatic. Tumor size was ranging from 0.4 to 20.0 cm (median, 5.5 cm), and two patients showed diffuse peritoneal tumor spread. In 15 patients (15%), the GIST was incidentally detected during major surgical procedures. In total, 44 GIST patients (44%) showed additional tumors (benign or malign), whereas in 36 patients (36%), the GIST occurred in coincidence with a malignant tumor (iso- or metachronic). Five patients (5%) had neurofibromatosis type 1. These results are also shown in Table 2.
Table 2.
Clinicopathologic Features.
| Initial symptoms (multiple mentions possible) | n | % of 101 |
| No clinical symptoms | 31 | 31% |
| Pain | 36 | 36% |
| GI bleeding | 28 | 28% |
| Anemia | 6 | 6% |
| Others | 25 | 25% |
| Localization of primary tumor | n | % of 99 |
| Stomach | 60 | 61% |
| Small bowel | 33 | 33% |
| Jejunum | 10 | 10% |
| Ileum | 18 | 18% |
| Duodenum | 5 | 5% |
| Colon | 1 | 1% |
| Esophagus | 2 | 2% |
| Others (EGIST, etc.) | 3 | 3% |
| Histomorphology | n | % of 101 |
| Spindle cell-like | 87 | 87% |
| Epithelioid | 2 | 2% |
| Mixed pattern | 12 | 11% |
| Risk of Malignancy (Fletcher et al.) | n (% of 95) | Gaster/Small Bowel/Other (n) |
| High risk | 37 (39%) | 20/12/3 |
| Intermediate risk | 20 (21%) | 14/5/1 |
| Low risk | 22 (23%) | 13/9/0 |
| Very low risk | 16 (17%) | 9/5/2 |
| Immunohistochemistry | Positive, n (%) | Negative, n (%) | Total, N |
| c-kit | 97 (97%) | 3 (3%) | 100 |
| p16 (10%) | 41 (43%) | 54 (57%) | 95 |
| P16 (20%) | 38 (40%) | 57 (60%) | 95 |
| P16 (50%) | 15 (16%) | 80 (84%) | 95 |
| CD34 | 66 (85%) | 12 (15%) | 78 |
| Aktin | 14 (21%) | 54 (79%) | 68 |
| Desmin | 4 (8%) | 44 (92%) | 48 |
| Vimentin | 32 (100%) | 0 (0%) | 32 |
| NSE | 7 (58%) | 5 (42%) | 12 |
| S100 | 1 (2%) | 56 (98%) | 57 |
EGIST indicates extra gastrointestinal stromal tumor; NSE, neuron-specific enolase.
Primary Tumor Site and Staging
The GISTs were predominantly located in the stomach (n = 60, 61%) and small intestine (n = 33, 33%; Table 2). Of the 101 patients, 14 (14%) had primary metastatic disease, 87 (86%) had only a locally limited primary growth. In total, 28 (28%) patients were affected by metastases or tumor recurrence at any time.
Surgical Treatment
Unless the local growth of the GIST itself or other additional malignancies required multivisceral resection, a local resection either of the stomach (wedge resection) or small intestine (segment resection) was performed. Dependent on the primary tumor site and the extent of tumor growth, complete resection could be achieved in 86% of the patients: in 88 patients, final resection state was R2 in 8 (9%), R1 in 4 (5%), and R0 in 76 (86%).
Metastases as well as Recurrence in GIST and Medical Treatment
In 101 patients, 14 (14%) had primary metastatic disease. A little more than one quarter of the patients (28/101, 28%) experienced recurrences or metastases within a median time of 1.93 years (range, 0.08–9.59 years; mean, 2.94 years). Thirteen patients were treated with imatinib (200–800 mg/day) in addition to surgery. Of these 13 patients, 6 (46%) achieved stable disease, 4 (31%) attained a partial remission, and 3 (23%) experienced tumor progression. Once, a neoadjuvant approach was realized within 15 weeks of preoperative application of imatinib (400 mg/day), which was continued after surgery. Imatinib did not lead to a complete remission in any of our patients.
Histopathologic and Pathoanatomic Analysis
Histomorphology of the GISTs was predominantly spindle cell-like (87/101, 86%). Of 101 GISTs, 2 (2%) were epithelioid-like and 12 tumors (12%) exhibited a mixed pattern of growth. Mitotic activity varied from apparently absent with no detectable mitotic figures in 50 HPFs to high (up to 89 mitotic figures/50 HPFs). According to Fletcher et al. [3] and Miettinen et al. [7], the risk of malignancy was classified as listed in Table 2. Tumor size ranged from 0.4 to 20 cm (median, 5.5 cm).
Immunohistochemical staining of the specimens (see also Table 2) showed c-Kit expression in 97% (97/101) of the tumors. CD34 expression was analyzed in 78 cases: 66 (85%) expressed CD34, whereby in 3 cases, CD34 was only detected at low levels in a minority of tumor cells. By using 10%, 20%, or 50% positive cells as cut-off value, the p16 expression was positive in 43%, 40%, or 16% and negative in 57%, 60%, or 84% of all analyzed tumors (95/101).
Outcome
Clinical follow-up of all surviving patients was carried out after a median interval of 4.13 years (range, 0.6–19 years; mean, 49.6 months; range, 7.2–228 months). Seventy-one patients (71% overall survival rate) are still alive, whereas 30 patients died (30% overall mortality rate), of which 14 (47%) were GIST-related deaths. The 1-, 3-, and 5-year GIST-related survival probability was 96%, 90%, and 85% (DSS) respectively. The overall recurrence rate was 28%. The 1-, 3-, and 5-year DFS probability counts for 81%, 77%, and 72% (DFS), respectively, are shown in Table 3.
Table 3.
Results of the Survival Analysis.
| 1-Year Probability (%) | 3-Year Probability (%) | 5-Year Probability (%) | |
| Overall | 91.9 | 80.8 | 69.5 |
| DSS | 95.9 | 89.7 | 84.9 |
| DFS | 80.8 | 77.1 | 72.2 |
| 1-Year DSS (%) | 3-Year DSS (%) | 5-Year DSS (%) | 1-Year DFS (%) | 3-Year DFS (%) | 5-Year DFS (%) | ||
| Prim-loc. | 100 | 98.6 | 93.0 | 94.0 | 89.6 | 83.9 | |
| Prim-met. | 70.1 | 35.1 | 35.1 | — | — | — | |
| Met: any time | 85.4 | 65.1 | 50.7 | — | — | — | |
| R0 resection | 97.3 | 95.7 | 89.1 | 88.0 | 84.8 | 82.8 | |
| R1/2 resection | 83.3 | 46.3 | — | 33.3 | 25.0 | — | |
| p16 (10%) | Positive | 94.7 | 84.8 | 80.5 | 79.9 | 79.9 | 72.0 |
| Negative | 96.3 | 92.1 | 86.8 | 79.3 | 75.1 | 72.5 | |
| p16 (20%) | Positive | 94.4 | 83.8 | 79.1 | 78.9 | 78.9 | 70.1 |
| Negative | 96.4 | 92.4 | 87.4 | 80.1 | 76.0 | 73.5 | |
| p16 (50%) | Positive | 92.3 | 73.8 | 63.3 | 66.0 | 66.0 | 56.6 |
| Negative | 96.2 | 91.7 | 87.9 | 81.1 | 79.1 | 74.8 | |
| Risk of malignancy | Very low | 100 | 100 | 100 | 100 | 100 | 100 |
| Low | 100 | 94.1 | 94.1 | 95.5 | 95.5 | 95.5 | |
| Intermediate | 100 | 100 | 100 | 100 | 100 | 100 | |
| High | 88.7 | 75.5 | 64.5 | 50.2 | 43.9 | 37.7 | |
| Non-high | 100.0 | 97.7 | 97.7 | 98.2 | 98.2 | 98.2 |
Met indicates metastases or recurrence at any time; Prim-loc, primarily localized; Prim-met, primarily metastases.
Dependency of Parameters
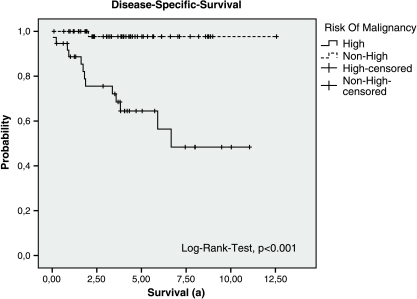
P values regarding the dependency of different parameters are listed in Table 4. The evaluations of survival predicting parameters are limited due to the small number of GIST-related deaths (14/101, 14%). However, tumor size and mitotic rate showed a statistically significant relation with the occurrence of metastases or recurrence as well as with the DFS rate and could be considered as clinically relevant. Also, risk classification (Figure 1) and the primary tumor state showed a relation with the occurrence of GIST-related death (yes/no), GIST-related survival rate, the occurrence of metastases or tumor recurrence (yes/no), and with the DFS.
Table 4.
P values.
| Independent Variables | Tumor-Related Death | DSS | DFS | Metastases/Recurrence | Count (n) |
| Fisher Exact/χ2 Test, P | Log-Rank Test, P | Log-Rank Test, P | Fisher Exact/χ2 Test, P | ||
| Sex | .779 | .657 | .724 | .665 | 101 |
| Localization (stomach vs small bowel) | 1.000 | .935 | .395 | .709 | 99 |
| Tumor size (≥5 cm vs <5 cm) | .005 | .008 | <.001 | <.001 | 94 |
| Mitotic rate (≥5/50 HPF vs <5/50 HPF) | .005 | .007 | <.001 | <.001 | 92 |
| Fletcher (high vs non-high) | <.001 | <.001 | <.001 | <.001 | 95 |
| Primary tumor state (unifocal vs not local) | <.001 | <.001 | <.001 | — | 101 |
| CD34 (positive vs negative) | 1.000 | .670 | .590 | 1.000 | 88 |
| Aktin (positive vs negative) | 1.000 | .611 | .236 | .319 | 68 |
| Desmin (positive vs negative) | 1.000 | .935 | .601 | 1.000 | 48 |
| p16 (positive vs negative, whole population) | |||||
| Cutoff > 10% | .980 | .818 | .897 | .764 | 95 |
| Cutoff > 20% | .813 | .596 | .745 | .926 | 95 |
| Cutoff > 50% | .226 | .080 | .207 | .279 | 95 |
| p16 (p16-positive high-risk GIST vs the rest) | |||||
| Cutoff > 10% | <.001 | <.001 | <.001 | <.001 | 95 |
| Cutoff > 20% | <.001 | <.001 | <.001 | <.001 | 95 |
| Cutoff > 50% | <.001 | <.001 | <.001 | <.001 | 95 |
P < .05 were considered significant (bold face emphases).
Figure 1.
Disease-specific survival of the groups “High-risk” and “Non-high-risk” (Very Low, Low, and Intermediate) modified according to Fletcher et al. (whole population).
In total, staining for p16 (only by taking a cutoff > 50%) was associated with poor outcome but statistically did not reach a level of relevance (P = .080, log-rank test; DSS).
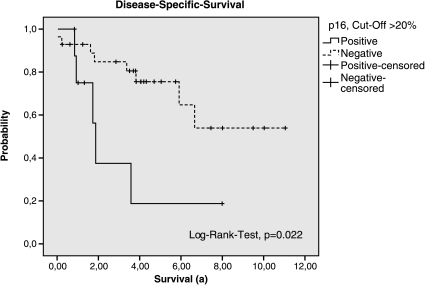
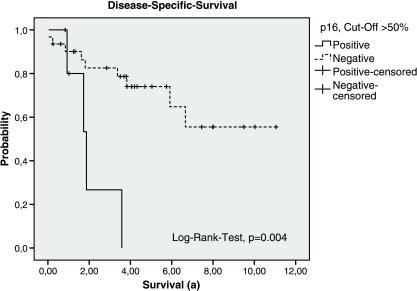
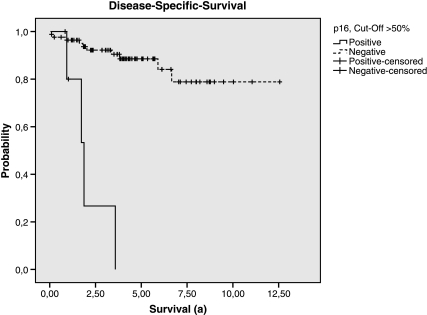
Therefore, in patients with high-risk GIST, the p16 expression was highly predictive for the reduction of DSS and DFS. Depending on the cutoff value, the P values in this subgroup were as follows: >10%, P = .118 (DSS) and P = .058 (DFS); >20%, P = .022 (DSS) and P = .006 (DFS); >50%, P = .004 (DSS) and P = .012 (DFS; Tables 5 and 6; Figures 2 and 3). Within the subgroup of patients who were affected by metastases or recurrence at any time, only the expression of p16 showed a statistically relevant relation with reduction of the survival (P = .041, log-rank test; cutoff, 50%).
Table 5.
Results of the Log-Rank Tests Concerning the Subgroups of High-Risk GISTs and Patients with Recurrence or Metastasis.
| p16 | High-Risk (n = 37) | Tumor Recurrence or Metastases | ||
| DSS | DFS | DSS | DFS | |
| Cutoff > 10% | P = .118 | P = .058 | P = .325 | P = .586 |
| Cutoff > 20% | P = .022 | P = .006 | P = .325 | P = .586 |
| Cutoff > 50% | P = .004 | P = .012 | P = .041 | P = .239 |
Table 6.
Disease-Specific and -Free Survival in High-Risk GIST.
| 1-Year Probability (%) | 3-Year Probability (%) | 5-Year Probability (%) | |
| Overall survival | 91.9 | 80.8 | 69.5 |
| DSS | 95.9 | 89.7 | 84.9 |
| DFS | 80.8 | 77.1 | 72.2 |
| High-Risk Patients (n = 37) | 1-Year DSS/DFS (%) | 3-Year DSS/DFS (%) | 5-Year DSS/DFS (%) |
| p16-positive | |||
| Cutoff > 10% | 77.8/30.0 | 46.7/30.0 | 31.1/20.0 |
| Cutoff > 20% | 75.0/22.2 | 37.5/22.2 | 18.8/11.1 |
| Cutoff > 50% | 80.0/16.7 | 26.7/16.7 | n.a./n.a. |
| p16-negative | |||
| Cutoff > 10% | 92.6/57.8 | 84.2/48.9 | 74.4/44.5 |
| Cutoff > 20% | 92.9/59.4 | 84.8/50.9 | 75.5/46.7 |
| Cutoff > 50% | 90.1/56.9 | 82.6/49.3 | 74.0/45.5 |
n.a. indicates not available.
Figure 2.
Disease-specific survival of the groups p16-positive and p16-negative (cutoff > 20%, subgroup of High-risk according to Fletcher et al.).
Figure 3.
Disease-specific survival of the groups p16-positive and p16-negative (cutoff > 50%, subgroup of High-risk according to Fletcher et al.).
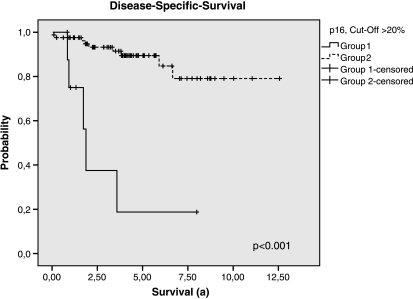
The most significant prognostic value was finally found by outcome analysis comparing the combination of immunohistochemical p16-positive high-risk GISTwith the remaining patients of the whole study population (all non-high-risk GIST regardless of p16 status and p16-negative high-risk GIST) as shown in Figures 4 and 5 and Table 4. The combination of p16 positivity and high-risk GIST showed the most highly predictive P values, independent of the cut-off values (DSS and DFS: P < .001, log-rank test; any cutoff value).
Figure 4.
Disease-specific survival of the groups 1 and 2 (group 1: High-risk GISTs, p16-positive (cutoff > 20%); group 2: High-risk GISTs, p16-negative and Non-high-risk GISTs regardless of p16 status).
Figure 5.
Disease-specific survival of the groups 1 and 2 (group 1: High-risk GISTs, p16-positive (cutoff > 50%); group 2: High-risk GISTs, p16-negative and Non.high-risk GISTs regardless of p16 status).
In multivariate analyses, no statistically relevant relating factors could be detected in addition.
Discussion
The aim of this study was to evaluate the prognostic value of p16 expression in GIST. Therefore, in 101 patients with GIST, the p16 immunostaining pattern of tumors was evaluated and statistically analyzed with regard to the dependency with clinicopathologic features, diagnostic appearance, and clinical outcome. Although high-risk classification is highly predictive in determining the probability of developing metastases or recurrence [3], this subgroup still remains heterogeneous. There exist no reliable parameters to further specify the individual risk of developing tumor recurrence, metastases, or GIST-related death. Furthermore, there are no significant predictors to assess the prognosis of GIST patients with tumor recurrence or metastases. Therefore, high-risk GIST and patients who were affected by metastases or tumor recurrence were specifically analyzed in individual subgroups to evaluate the prognostic value of p16 expression in these cohorts.
Of the 101 patients, GIST in 15 (15%) was accidentally detected during major surgery. Furthermore, in clinical presentation, the leading symptoms were abdominal pain (36%) and GI bleeding (28%), which are higher in comparison to other published data where bowel obstruction (15%) and perforation (10%) are the major problems [9,23–26]. These were only found in a few cases in our study. Similarly, the distribution of the risk classification differs. In contrast to Orosz et al. [27], who reported an 80% rate of high-risk tumors within a study of 136 patients, we found a rate of only 39% (Table 2). One explanation for this discrepancy might be that the diagnosis was made at an earlier stage in our patients. As suggested previously, such data could vary (reviewed in Rubin et al. [28]), and this highlights the limited comparability of different populations.
Interestingly, in comparison to other studies [5,23–26,29–39], we report the highest coincidence rate concerning additional malignancies in GIST (36/101, 36%). Since 2000, the coincidence rate of malignancies in GIST is described in several studies ranging between 4.5% and 26.8% while the study size encompasses from 18 to 747 GIST patients [5,23–26,29–39]. This leads to a mean rate of 10% for additional malignancies in GIST patients. Whether there might be a biologic interaction related to the coincidence of GIST and additional neoplasia remains to be further investigated.
In our study, approximately one quarter of the GIST patients (28/101, 28%) were affected by recurrence or metastases and half of these (14%) had primary metastatic GIST disease. This is low compared to a 50% rate published by two other groups [6,35]. This difference might be explained by the selection of patients due to the different character of the medical or surgical center. Nevertheless, our results are supported by the findings of three studies reporting a 10% to 25% rate of patients with metastatic disease [36,40,41].
Of course, the outcome of patients after R1/R2 resection was worse in comparison to R0 resection. These results are in accordance with population-based studies reporting a median survival of 68 months for complete resection, 51 months for partial resection, and 10 months for no resection each with P ≤ .001. Outcomes in patients with completely resected tumors were found to be equivalent regardless of whether they had wedge or total organ removal [23,42–44].
Obviously, concerning medical treatment, the number of only 13 patients who were treated with imatinib in our series does not permit statistical conclusions. In general, response rates of 50% to 80% are reported after treatment with imatinib, whereas partial remission in 40% to 50% of these patients is seen, and in one third, a stable state might be achievable [5,6,40,43,44]. This is in accordance with our data.
The 1-, 3-, and 5-year GIST-related survival probability of our patients is 96%, 90%, and 85% (DSS), respectively. Whereas the overall recurrence rate is 28%, the 1-, 3-, and 5-year DFS probability counts for 81%, 77%, and 72%, respectively (Table 3). Taken together, the GIST survival rates of our patients seem to be rather high in comparison to other published data [6,32,41,44,45].
Our immunohistochemical analysis revealed a profound expression of c-Kit in 97% (97/101) and of CD34 in 85% (66/78) of the tumors. These results (Table 2) are in accordance with those of previously published studies [3,27,46]. With regards to the whole population of our study, the expression of p16 in GIST tends to result in predicting poor outcome, but a statistically relevant level is not reached (P = .08, log-rank test - DSS; cutoff, 50%). Whereas in patients with high-risk GIST, the p16 expression was highly predictive for the reduction of DFS and DSS (P = .012 and P = .004; cutoff, 50%). Depending on the cutoff value, the P values are listed in Table 5. In addition, in our study, the expression of p16 is the only statistically relevant, thus highly predictive parameter regarding the reduction of the DSS of GIST patients who were affected by metastases or recurrence (P = .041; Table 5). Therefore, p16 staining might be used as predictor.
However, at present, it is still difficult to estimate the predicting value of p16 in GIST, although this issue has been addressed in several publications [14–22,47]. Because different methods, different cutoff values, different follow-up durations, and different searching variables were used, the comparability of these data is limited. Most studies describe statistically relevant relations/associations only with malignant risk classification [14–22,47] and not with the GIST-related prognosis itself. Beside the here-presented data, the results of only three additional studies [14,21,22] indicate a predictive value of p16 expression in GIST, if P < .05 is considered statistically significant.
In contrast to the data presented in our study, the loss of p16 was described twice as significantly predictive for poor outcome in GIST (P < .02, n = 39 and P = .012, n = 159; cutoff, 20%) [14,21]. However, the according duration of follow-up was only 34 [21] or 45 months [14], and the average center size was less than 39 patients in these studies, whereas in our single-center population, the mean follow-up duration was 54 months (range, 3–229 months) and, within the subgroup of the surviving patients, even 61 months (range, 7–229 months), thus at least 9 months longer. This emphasizes the role of a sufficient follow-up duration. Certainly, only additional long-term follow-up, within a further couple of years, may clarify the relevance of the p16 expression in GIST.
The results of a third study are in accordance with our data. Steigen et al. [22] also found a positive relation between p16 staining and poor prognosis in GIST (n = 434, P = .013). Unfortunately, the median follow-up duration is not documented in their article [22], and their data were acquired out of 20 departments of pathology in Norway (average center size, ∼22 patients per center). Thus, our study represents the largest single-center evaluation of p16 immunostaining in GIST by including 101 patients with the longest mean follow-up time, counting 54 months.
However, which pathophysiological role p16 might play in the oncogenesis of GIST remains to be further investigated. It might be even different at certain stages of tumorigenesis. Actually, the loss of p16 expression is biologically contributed to malignancy. However, if other oncogenic changes such as loss of RB or TP53 and aberrant activation of cyclin D by gene amplification or by activating mutations of the receptor tyrosine kinase ras-raf-ERK lead to highly increased proliferation, the expression of p16 might also be a compensatory reaction on oncogenic escalation of other origin. Indeed, it is known that in cervical carcinomas the expression of p16 contributes to malignancy [48,49] and in breast cancer even to poor outcome [50]. Just recently, also in GIST the relation between p16 expression and the mitotic rate was confirmed [22].
The prognostic value of p16 in our study was also characterized applying the more recently published Hornick and Fletcher classification [51], which is based on the Miettinen and Lasota classification [52]. Interestingly, the prognostic value of p16 in our study appeared even more significant when using the Hornick and Fletcher classification (data not shown). In patients with high-risk GIST, the p16 expression was highly predictive for the reduction of DSS and DFS by using the Hornick and Fletcher classification (range, P < .002 to P < .056). However, to the best of our knowledge, the risk classification of Fletcher is still the accepted one, which was discussed by the World Health Organization Consensus Conference in 2001. As long as the risk classification of Hornick and Fletcher [51] is not accepted within the context of the international, interdisciplinary consensus conferences, we prefer to accord to the well-established Fletcher consensus classification.
The conclusion of our study can be summarized as follows: the major clinical symptoms in GIST are abdominal pain and GI bleeding. To the best of our knowledge, we report the highest coincidence of additional malignancies with GIST (36%). Primary tumor state high-risk classification and the combination, “p16-positive high-risk GIST,” are the statistically most relevant predictors for poor prognosis. In high-risk GIST, the expression of p16 is highly predictive for the reduction of the DFS and DSS. In patients with recurrence or metastases, the expression of p16 seems to be the only clinical relevant predictor. Therefore, p16 staining allows to differentiate high-risk from “very high-risk” GIST and might be used as predictor.
Acknowledgments
The authors thank Suzanne Gaskell (Preston, Great Britain) and Annette Blatz for editorial assistance and Beate Rimmel for technical assistance.
Abbreviations
- DFS
disease-free survival
- DSS
disease-specific survival
- GI
gastrointestinal
- GIST
gastrointestinal stromal tumor
- HPF
high-power field
Footnotes
No contents of the manuscript have been submitted or published elsewhere. All authors have contributed to the work and approved the final version of the manuscript.
Conflict of interest
There are no conflicts of interest to disclose.
References
- 1.Kindblom LG, Meis-Kindblom JM, Bumming P. Incidence, prevalence, phenotype and biologic spectrum of gastrointestinal stromal cell tumors (GIST)—a population-based study of 600 cases. Ann Oncol. 2002;13:157. [Google Scholar]
- 2.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 4.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 5.Crosby JA, Catton CN, Davis A, Couture J, O'Sullivan B, Kandel R, Swallow CJ. Malignant gastrointestinal stromal tumors of the small intestine: a review of 50 cases from a prospective database. Ann Surg Oncol. 2001;8:50–59. doi: 10.1007/s10434-001-0050-4. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg BL, Judson I. Surgery and imatinib in the management of GIST: emerging approaches to adjuvant and neoadjuvant therapy. Ann Surg Oncol. 2004;11:465–475. doi: 10.1245/ASO.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Miettinen M, El-Rifai W, HL Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478–483. doi: 10.1053/hupa.2002.124123. [DOI] [PubMed] [Google Scholar]
- 8.van Oosterom AT, Judson IR, Verweij J, Stroobants S, Dumez H, Donato di Paola E, Sciot R, Van Glabbeke M, Dimitrijevic S, Nielsen OS. Update of phase I study of imatinib (STI571) in advanced soft tissue sarcomas and gastrointestinal stromal tumors: a report of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2002;38(Suppl 5):S83–S87. doi: 10.1016/s0959-8049(02)80608-6. [DOI] [PubMed] [Google Scholar]
- 9.Esteller M, Gonzalez S, Risques RA, Marcuello E, Mangues R, Germa JR, Herman JG, Capella G, Peinado MA. K-ras and p16 aberrations confer poor prognosis in human colorectal cancer. J Clin Oncol. 2001;19:299–304. doi: 10.1200/JCO.2001.19.2.299. [DOI] [PubMed] [Google Scholar]
- 10.Gessner C, Liebers U, Kuhn H, Stiehl P, Witt C, Schauer J, Wolff G. BAX and p16INK4A are independent positive prognostic markers for advanced tumour stage of nonsmall cell lung cancer. Eur Respir J. 2002;19:134–140. doi: 10.1183/09031936.02.00219402. [DOI] [PubMed] [Google Scholar]
- 11.Hilton DA, Penney M, Evans B, Sanders H, Love S. Evaluation of molecular markers in low-grade diffuse astrocytomas: loss of p16 and retinoblastoma protein expression is associated with short survival. Am J Surg Pathol. 2002;26:472–478. doi: 10.1097/00000478-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi K, Oda Y, Saito T, Yamamoto H, Tamiya S, Takahira T, Miyajima K, Iwamoto Y, Tsuneyoshi M. Mechanisms of inactivation of the p16INK4a gene in leiomyosarcoma of soft tissue: decreased p16 expression correlates with promoter methylation and poor prognosis. J Pathol. 2003;201:487–495. doi: 10.1002/path.1419. [DOI] [PubMed] [Google Scholar]
- 13.Makitie AA, MacMillan C, Ho J, Shi W, Lee A, O'Sullivan B, Payne D, Pintilie M, Cummings B, Waldron J, et al. Loss of p16 expression has prognostic significance in human nasopharyngeal carcinoma. Clin Cancer Res. 2003;9:2177–2184. [PubMed] [Google Scholar]
- 14.Schneider-Stock R, Boltze C, Lasota J, Peters B, Corless CL, Ruemmele P, Terracciano L, Pross M, Insabato L, Di Vizio D, et al. Loss of p16 protein defines high-risk patients with gastrointestinal stromal tumors: a tissue microarray study. Clin Cancer Res. 2005;11:638–645. [PubMed] [Google Scholar]
- 15.Haller F, Gunawan B, von Heydebreck A, Schwager S, Schulten HJ, Wolf-Salgo J, Langer C, Ramadori G, Sultmann H, Fuzesi L. Prognostic role of E2F1 and members of the CDKN2A network in gastrointestinal stromal tumors. Clin Cancer Res. 2005;11:6589–6597. doi: 10.1158/1078-0432.CCR-05-0329. [DOI] [PubMed] [Google Scholar]
- 16.Huang HY, Huang WW, Lin CN, Eng HL, Li SH, Li CF, Lu D, Yu SC, Hsiung CY. Immunohistochemical expression of p16INK4A, Ki-67, and Mcm2 proteins in gastrointestinal stromal tumors: prognostic implications and correlations with risk stratification of NIH consensus criteria. Ann Surg Oncol. 2006;13:1633–1644. doi: 10.1245/s10434-006-9188-4. [DOI] [PubMed] [Google Scholar]
- 17.Perrone F, Tamborini E, Dagrada GP, Colombo F, Bonadiman L, Albertini V, Lagonigro MS, Gabanti E, Caramuta S, Greco A, et al. 9p21 locus analysis in high-risk gastrointestinal stromal tumors characterized for c-kit and platelet-derived growth factor receptor alpha gene alterations. Cancer. 2005;104:159–169. doi: 10.1002/cncr.21113. [DOI] [PubMed] [Google Scholar]
- 18.Ricci R, Arena V, Castri F, Martini M, Maggiano N, Murazio M, Pacelli F, Potenza AE, Vecchio FM, Larocca LM. Role of p16/INK4a in gastrointestinal stromal tumor progression. Am J Clin Pathol. 2004;122:35–43. doi: 10.1309/MJ4X-N2M5-7HNC-8X5H. [DOI] [PubMed] [Google Scholar]
- 19.Sabah M, Cummins R, Leader M, Kay E. Loss of heterozygosity of chromosome 9p and loss of p16INK4A expression are associated with malignant gastrointestinal stromal tumors. Mod Pathol. 2004;17:1364–1371. doi: 10.1038/modpathol.3800199. [DOI] [PubMed] [Google Scholar]
- 20.Sabah M, Cummins R, Leader M, Kay E. Altered expression of cell cycle regulatory proteins in gastrointestinal stromal tumors: markers with potential prognostic implications. Hum Pathol. 2006;37:648–655. doi: 10.1016/j.humpath.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Schneider-Stock R, Boltze C, Lasota J, Miettinen M, Peters B, Pross M, Roessner A, Gunther T. High prognostic value of p16INK4 alterations in gastrointestinal stromal tumors. J Clin Oncol. 2003;21:1688–1697. doi: 10.1200/JCO.2003.08.101. [DOI] [PubMed] [Google Scholar]
- 22.Steigen SE, Bjerkehagen B, Haugland HK, Nordrum IS, Loberg EM, Isaksen V, Eide TJ, Nielsen TO. Diagnostic and prognostic markers for gastrointestinal stromal tumors in Norway. Mod Pathol. 2008;21:46–53. doi: 10.1038/modpathol.3800976. [DOI] [PubMed] [Google Scholar]
- 23.Hohenberger P, Reichardt P, Gebauer B, Wardelmann E. Gastrointestinal stromal tumors (GIST)—current concepts of surgical management. Dtsch Med Wochenschr. 2004;129:1817–1820. doi: 10.1055/s-2004-829035. [DOI] [PubMed] [Google Scholar]
- 24.Iesalnieks I, Rummele P, Dietmaier W, Jantsch T, Zulke C, Schlitt HJ, Hofstadter F, Anthuber M. Factors associated with disease progression in patients with gastrointestinal stromal tumors in the pre-imatinib era. Am J Clin Pathol. 2005;124:740–748. doi: 10.1309/AKK3-VFF6-10CW-M566. [DOI] [PubMed] [Google Scholar]
- 25.Kim KM, Kang DW, Moon WS, Park JB, Park CK, Sohn JH, Jeong JS, Cho MY, Jin SY, Choi JS, et al. Gastrointestinal stromal tumors in Koreans: it's incidence and the clinical, pathologic and immunohistochemical findings. J Korean Med Sci. 2005;20:977–984. doi: 10.3346/jkms.2005.20.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liszka L, Zielinska-Pajak E, Pajak J, Golka D, Huszno J. Coexistence of gastrointestinal stromal tumors with other neoplasms. J Gastroenterol. 2007;42:641–649. doi: 10.1007/s00535-007-2082-4. [DOI] [PubMed] [Google Scholar]
- 27.Orosz Z, Tornoczky T, Sapi Z. Gastrointestinal stromal tumors: a clinicopathologic and immunohistochemical study of 136 cases. Pathol Oncol Res. 2005;11:11–21. doi: 10.1007/BF03032400. [DOI] [PubMed] [Google Scholar]
- 28.Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–1741. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- 29.Agaimy A, Wunsch PH, Sobin LH, Lasota J, Miettinen M. Occurrence of other malignancies in patients with gastrointestinal stromal tumors. Semin Diagn Pathol. 2006;23:120–129. doi: 10.1053/j.semdp.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Au WY, Ho KM, Shek TW. Papillary renal cell carcinoma and gastrointestinal stromal tumor: a unique association. Ann Oncol. 2004;15:843–844. doi: 10.1093/annonc/mdh191. [DOI] [PubMed] [Google Scholar]
- 31.Boni L, Benevento A, Dionigi G, Rovera F, Dionigi R. Surgical resection for gastrointestinal stromal tumors (GIST): experience on 25 patients. World J Surg Oncol. 2005;3:78. doi: 10.1186/1477-7819-3-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassan I, You YN, Dozois EJ, Shayyan R, Smyrk TC, Okuno SH, Donohue JH. Clinical, pathologic, and immunohistochemical characteristics of gastrointestinal stromal tumors of the colon and rectum: implications for surgical management and adjuvant therapies. Dis Colon Rectum. 2006;49:609–615. doi: 10.1007/s10350-006-0503-8. [DOI] [PubMed] [Google Scholar]
- 34.Maiorana A, Fante R, Maria Cesinaro A, Adriana Fano R. Synchronous occurrence of epithelial and stromal tumors in the stomach: a report of 6 cases. Arch Pathol Lab Med. 2000;124:682–686. doi: 10.5858/2000-124-0682-SOOEAS. [DOI] [PubMed] [Google Scholar]
- 35.Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625–641. doi: 10.1097/00000478-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 37.Ruka W, Rutkowski P, Nowecki Z, Nasierowska-Guttmejer A, Debiec-Rychter M. Other malignant neoplasms in patients with gastrointestinal stromal tumors (GIST) Med Sci Monit. 2004;10:LE13–LE14. [PubMed] [Google Scholar]
- 38.Tornoczky T, Kover E. Multiple soft tissue sarcomas. Cancer. 2005;104:440–441. doi: 10.1002/cncr.21176. author reply 441. [DOI] [PubMed] [Google Scholar]
- 39.Wronski M, Ziarkiewicz-Wroblewska B, Gornicka B, Cebulski W, Slodkowski M, Wasiutynski A, Krasnodebski IW. Synchronous occurrence of gastrointestinal stromal tumors and other primary gastrointestinal neoplasms. World J Gastroenterol. 2006;12:5360–5362. doi: 10.3748/wjg.v12.i33.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655–664. doi: 10.1016/s1470-2045(02)00899-9. [DOI] [PubMed] [Google Scholar]
- 41.Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100:162–168. doi: 10.1111/j.1572-0241.2005.40709.x. [DOI] [PubMed] [Google Scholar]
- 42.Langer C, Gunawan B, Schuler P, Huber W, Fuzesi L, Becker H. Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours. Br J Surg. 2003;90:332–339. doi: 10.1002/bjs.4046. [DOI] [PubMed] [Google Scholar]
- 43.Perez EA, Gutierrez JC, Jin X, Lee DJ, Rocha-Lima C, Livingstone AS, Franceschi D, Koniaris LG. Surgical outcomes of gastrointestinal sarcoma including gastrointestinal stromal tumors: a population-based examination. J Gastrointest Surg. 2007;11:114–125. doi: 10.1007/s11605-006-0072-0. [DOI] [PubMed] [Google Scholar]
- 44.Pierie JP, Choudry U, Muzikansky A, Yeap BY, Souba WW, Ott MJ. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg. 2001;136:383–389. doi: 10.1001/archsurg.136.4.383. [DOI] [PubMed] [Google Scholar]
- 45.Rubio J, Marcos-Gragera R, Ortiz MR, Miro J, Vilardell L, Girones J, Hernandez-Yague X, Codina-Cazador A, Bernado L, Izquierdo A, et al. Populationbased incidence and survival of gastrointestinal stromal tumours (GIST) in Girona, Spain. Eur J Cancer. 2007;43:144–148. doi: 10.1016/j.ejca.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura N, Yamamoto H, Yao T, Oda Y, Nishiyama K, Imamura M, Yamada T, Nawata H, Tsuneyoshi M. Prognostic significance of expressions of cell-cycle regulatory proteins in gastrointestinal stromal tumor and the relevance of the risk grade. Hum Pathol. 2005;36:828–837. doi: 10.1016/j.humpath.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Cameron RI, Maxwell P, Jenkins D, McCluggage WG. Immunohistochemical staining with MIB1, bcl2 and p16 assists in the distinction of cervical glandular intraepithelial neoplasia from tubo-endometrial metaplasia, endometriosis and microglandular hyperplasia. Histopathology. 2002;41:313–321. doi: 10.1046/j.1365-2559.2002.01465.x. [DOI] [PubMed] [Google Scholar]
- 49.O'Neill CJ, McCluggage WG. p16 expression in the female genital tract and its value in diagnosis. Adv Anat Pathol. 2006;13:8–15. doi: 10.1097/01.pap.0000201828.92719.f3. [DOI] [PubMed] [Google Scholar]
- 50.Munot K, Bell SM, Lane S, Horgan K, Hanby AM, Speirs V. Pattern of expression of genes linked to epigenetic silencing in human breast cancer. Hum Pathol. 2006;37:989–999. doi: 10.1016/j.humpath.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 51.Hornick JL, Fletcher CDM. The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol. 2007;38:679–687. doi: 10.1016/j.humpath.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]