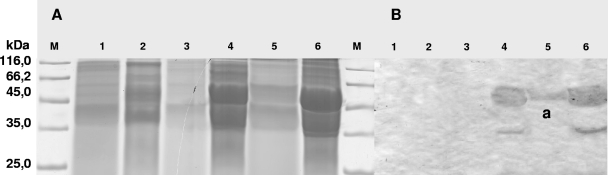
FIG. 7.
Immunological detection of Lcp from E. coli/pET23a::hyaAlcpK30 by Western blotting. (A) Electropherogram of an SDS-polyacrylamide gel after separation of proteins from crude cell extracts and extracellular protein fractions. Proteins in the gel were stained with Coomassie brilliant blue R250. (B) Western blot employing anti-LcpK30 IgGs prepared from an SDS-polyacrylamide gel. Lane M, molecular mass standard; lane 1, extracellular protein fraction of E. coli harboring pET23a; lane 2, crude cell extract of E. coli harboring pET23a; lane 3, extracellular protein fraction of E. coli C41(DE3) harboring pET23a::hyaAlcpK30; lane 4, crude cell extract of E. coli C41(DE3) harboring pET23a::hyaAlcpK30; lane 5, extracellular protein fraction of E. coli C43(DE3) harboring pET23a::hyaAlcpK30; lane 6, crude cell extract of E. coli C43(DE3) harboring pET23a::hyaAlcpK30. The E. coli cultures were grown in LB medium at 37°C to an optical density at 600 nm of 0.5, and then expression was induced by addition of IPTG to a final concentration of 1 mM for 3 h, which resulted in cells with IBs. In the Western blot the anti-LcpK30 IgGs recognized the approximately 43-kDa LcpK30 protein (band a).