Abstract
Improved methods for selective isolation of diverse actinomycetes of the genus Micromonospora and a genus-specific nested PCR for rapid identification of putative Micromonospora isolates were developed. The robustness of both the isolation and the identification approach was underpinned by phylogenetic analysis based on 16S rRNA gene sequences.
The genus Micromonospora is a prolific source of various bioactive metabolites, such as antibiotics and enzyme inhibitors (1, 7, 10, 11, 23), second only to Streptomyces in the order Actinomycetales (3). At the time of writing, there were 34 valid species in this genus. Members of Micromonospora are widely distributed in a variety of habitats, notably soil rich in humus, and play an important role in the decomposition of organic matter, but they are usually not the preponderant components of the actinomycete population (6, 8, 9, 27, 30). Hayakawa et al. (9) devised a method effective for the selective isolation of this genus by pretreating soil samples with 1.5% phenol and using humic acid-vitamin (HV) agar as a basal isolation medium. However, in practical use, colonies are difficult to observe on the dark HV agar plates, especially the small colonies bearing no aerial mycelium and sporulating slowly, which are typical culture characteristics of Micromonospora (14, 30). To reduce the number of nonfilamentous bacteria, heating dried soil was effective (26). The antibiotic novobiocin has been used to improve the selectivity of isolation media for some rare actinomycete genera (4, 27). In an effort to facilitate the isolation of Micromonospora strains for biotechnological exploitation and to gain better understanding of their diversity, we developed in this study improved effective methods for isolating these organisms from soil.
Members of the genus Micromonospora are not easy to identify on the basis of morphology, for they may form indistinct colonies that can be confused with other actinomycetes and are similar in microscopic cell morphology to some other genera in the family Micromonosporaceae. Compared to the tedious conventional morphological and time-consuming chemical techniques, PCR-based detection using genus-specific primers has proved useful for rapid identification of several actinomycete genera (18, 21, 22, 24, 29, 31). The specific PCR primer set for the family Micromonosporaceae has also been reported and used for some years (19); this set, however, was found to be not very specific in our preliminary studies, as it also amplified sequences from Streptomyces spp. Thus, another aim of the present study was to design a pair of Micromonospora genus-specific primers for rapid identification of putative Micromonospora isolates and for monitoring the effectiveness of the isolation procedures we developed.
Humus-rich soil samples were collected from three locations in the virgin forest of Kanas Nature Reserve, Xinjiang, northwest China, in August 2006 and from four locations in mangrove forests in Hainan Province, south China, in October 2006. A clean sampling scoop was used to take samples from 5- to 20-cm depths, and samples were air dried at room temperature for 1 month and were triturated by using a sterile pestle. Three to five samples from different depths of each location were then mixed, respectively, to form seven composite samples, each of which represented one site and was further processed using the three methods described below.
In method 1 (ultrasonication/dilution), 1 g of the composite sample was added to 9 ml sterile water. The resulting 10−1 dilution was shaken for 2 h at room temperature and 180 rpm and then ultrasonicated in a water bath sonicator (model KQ-100DB, 40 kHz, 100 W; Kunshan Ultrasonic instruments Co., Ltd., Kunshan, China) for 2 min at 30°C and further diluted (1:10) down to 10−4 with sterile water.
In method 2 (1.5% phenol), the 10−1 sample dilution was vigorously shaken for 5 min on a vortex mixer and then treated by ultrasonication as described for method 1. The suspension was treated with 1.5% phenol as previously described (9) and further diluted (1:10) down to 10−4 with sterile water.
In method 3 (wet heat), 1 ml of the ultrasonic-treated 10−1 dilution prepared as described for method 2 was diluted (1:10) down to 10−4 with sterile water. The dilutions were then heated at 100°C for 1 h.
Amounts of 200 μl of each of the pretreated 10−2, 10−3, and 10−4 dilutions were spread in triplicate over the surface of 5 to 13 solid media that were designed for cultivation of actinomycetes: oatmeal agar (ISP 3; DSMZ medium 609), glycerol-asparagine agar (ISP 5; DSMZ medium 993), GYM (DSMZ medium 65), soil extract agar (SEA; DSMZ medium 12), Gause inorganic agar (5), modified starch casein agar (15), Sauton's agar (20), DNB agar (DNBA) (12), DNB gellan gum (12), HV agar (HVA) (8), HV gellan gum (HVG), glycerol-arginine agar (GA) (16), and calcium malic acid agar (CM; 10.0 g calcium malic acid, 10.0 g glycerol, 0.5 g NH4Cl, 0.05 g K2HPO4, 15 g agar, 1,000 ml distilled water). All media contained cycloheximide, nystatin, and nalidixic acid (each at 50 μg/ml) and were amended with or without novobiocin (25 μg/ml). The initial pH of each medium was controlled at 7.2 to 7.4.
All plates were incubated at 28°C for 3 to 4 weeks. Colonies that showed cultural characteristics of the genus Micromonospora (14, 30) were counted, as well as colonies of nonactinomycete bacteria and other actinomycetes. Micromonospora-like representatives of each colony morphotype from each of the plates with HVA, ISP 3, and DNBA and pretreatment methods 2 and 3 were picked out and purified on GYM plates. The morphology of hyphae and spores was observed by light microscopy using the coverslip technique (13) and by scanning electron microscopy.
Genus-specific primers for Micromonospora strains were derived from the 16S rRNA gene sequence. Available sequences of all valid species within the family Micromonosporaceae, together with 12 valid species of other families and several informally described species within and outside of the genus Micromonospora (see Table S1 in the supplemental material), were aligned in MEGA 4.0 (28) using the ClustalW algorithm to determine the regions conserved only among members of Micromonospora. Primer Premier 5.0 (Premier Biosoft International), BLASTN (NCBI), and Probe Match (RDP) programs and SPCR 3.0 (http://moleco.sjtu.edu.cn/moleco/softwares.html) were used to design and evaluate the primers. A nested-PCR approach was developed as a rapid method to identify strains that belong to the genus Micromonospora. The 16S rRNA gene was first amplified from genomic DNA by using universal primers 27f and 1492r (17). A 1:100 dilution of the resulting PCR product was used as a template for the second amplification. A 50-μl amount of the reaction mixture contained 1 μl template DNA, 5 μl 10× PCR buffer [100 mM KCl, 80 mM (NH4)2SO4, 100 mM Tris-HCl (pH 9.0), 0.5% NP-40], 2.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphate mixture, 0.5 μM each of the Micromonospora genus-specific primers M558F and C1028R, 2.5 U Taq DNA polymerase, and 10% dimethyl sulfoxide. The conditions consisted of an initial denaturation at 95°C for 4 min; 35 cycles at 95°C for 45 s, 68.5°C for 45 s, and 72°C for 1 min; and a final 8-min extension at 72°C.
In order to assess the identities of the strains isolated, the nearly full-length sequences of the 16S rRNA gene were analyzed. PCR products of the first amplification were purified and directly sequenced by using an Applied Biosystems DNA sequencer (model 3730XL) and software provided by the manufacturer. The resulting 16S rRNA gene sequences (>1,380 nucleotides) were used to search the GenBank database with the BLASTN program to determine relative phylogenetic positions. Phylogenetic analysis was conducted using MEGA 4.0 (28) by first generating a complete alignment of 16S rRNA gene sequences of the isolates and type strains of all valid species within the genus Micromonospora, with the exception of Micromonospora gallica, whose 16S rRNA sequence is not available from public databases, and then inferring neighbor-joining tree and bootstrap values. The phylogenetic diversity of the isolates was estimated by performing cluster analysis. A phylotype was defined as a group of strains exhibiting ≥99% 16S rRNA gene sequence similarity and was considered undescribed if all strains within this phylotype shared <99% sequence similarity with any of the type strains (6).
In order to focus on relatively effective media for selective isolation, one composite sample from Kanas Lake Reserve and one from the mangrove forest were first processed using all the media and the method 1 pretreatment for trials. According to the results shown in Table 1, we applied five media which gave higher ratios of Micromonospora-like colonies, i.e., HVA, ISP 3, DNBA, GA, and CM amended with cycloheximide, nystatin, nalidixic acid, and novobiocin, to all composite samples pretreated by the three methods.
TABLE 1.
Average numbers of colonies recorded on different media seeded with two composite samples processed by ultrasonication/dilution pretreatment (method 1)
| Mediuma | No. of colonies (avg ± SD [104 CFU/g dry weight composite soil]) of:
|
||
|---|---|---|---|
| Nonactinomycete bacteria | Micromonospora-like actinomycetes | Other actinomycetes | |
| GYM | 93.7 ± 16.3 | 0 | 2.0 ± 0.6 |
| Gause inorganic agar | 66.7 ± 15.0 | 0 | 0.4 ± 0.3 |
| Glycerol asparagine agar | 1.0 ± 0.6 | 0 | 0 |
| Modified starch casein agar | 9.3 ± 2.1 | 0 | 2.3 ± 0.6 |
| Sauton's agar | 34.5 ± 3.2 | 0 | 15.0 ± 0.7 |
| DNB gellan gum | 21.6 ± 1.7 | 0 | 0.01 ± 0.003 |
| SEA | 17.3 ± 0.6 | 0.01 ± 0.003 | 3.0 ± 1.5 |
| SEA plus NB | 6.7 ± 0.3 | 0.03 ± 0.01 | 2.0 ± 0.01 |
| DNBA | 10.0 ± 4.6 | 0.01 ± 0.003 | 0.01 ± 0.003 |
| DNBA plus NB | 4.3 ± 1.5 | 0.02 ± 0.01 | 0.01 ± 0.003 |
| ISP 3 | 32.0 ± 5.8 | 0.5 ± 0.3 | 1.0 ± 0.9 |
| ISP 3 plus NB | 17.7 ± 3.7 | 0.8 ± 0.3 | 0.4 ± 0.1 |
| HVA | 26.5 ± 1.5 | 0.2 ± 0.1 | 2.5 ± 1.5 |
| HVA plus NB | 17.5 ± 2.1 | 0.4 ± 0.1 | 0.7 ± 0.6 |
| HVG | 33.0 ± 0.6 | 0.01 ± 0.003 | 0.8 ± 0.6 |
| HVG plus NB | 11.0 ± 0.5 | 0.2 ± 0.03 | 0.9 ± 0.2 |
| GA | 3.0 ± 1.5 | 0.01 ± 0.05 | 0.3 ± 0.1 |
| GA plus NB | 0.8 ± 0.5 | 0.01 ± 0.003 | 0 |
| CM | 3.0 ± 1.5 | 0.2 ± 0.1 | 0.5 ± 0.4 |
| CM plus NB | 2.3 ± 0.7 | 0.4 ± 0.1 | 0.2 ± 0.3 |
All media contained cycloheximide, nystatin, and nalidixic acid (each at 50 μg/ml). NB, novobiocin at a concentration of 25 μg/ml.
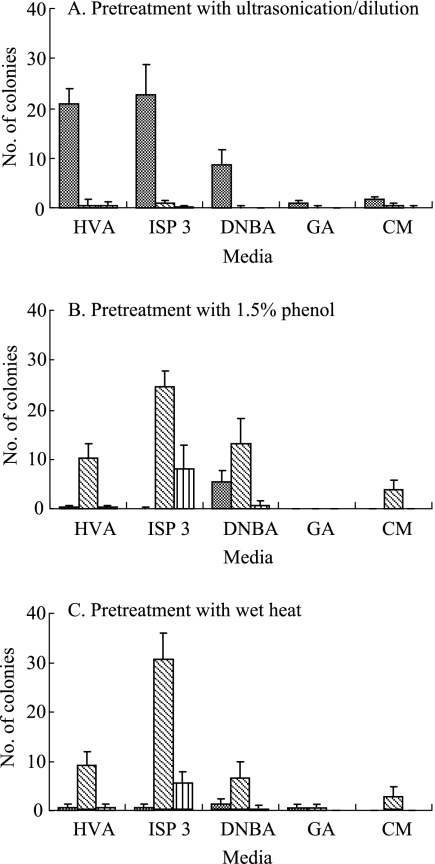
Pretreatment with 1.5% phenol or wet heat drastically reduced the numbers of nonactinomycete bacterial colonies (P < 0.01) while significantly increasing the numbers of Micromonospora-like colonies (P < 0.05) (Fig. 1). No matter which pretreatment method was applied, HVA, ISP 3, and DNBA media always gave better isolation effects than other media in both selectivity (percentage of Micromonospora-like colonies) and yield (number of Micromonospora-like colonies), and this was observed for all the composite samples we studied. HVA medium gave higher selectivity than ISP 3 and DNBA, but its average yield was over two- to threefold less than that of ISP 3, as shown in Fig. 1B and C. Despite the fact that ISP 3 medium also increased the recovery of other actinomycetes, combining it with wet-heat pretreatment gave a rich diversity of Micromonospora-like colonies, outstanding selectivity, and a much higher yield than any of the other combinations of medium and pretreatment, thus proving this to be the most-robust approach for selective isolation of Micromonospora. Moreover, the wet-heat method is very simple and easy to apply, and Micromonospora colonies grown on ISP 3 were much more easily observed and then picked.
FIG. 1.
Average numbers of colonies (104 CFU/g dry weight composite soil) of nonactinomycete bacteria (grey bars), Micromonospora-like actinomycetes (hatched bars), and other actinomycetes (striped bars) recorded on different selective plates seeded with all seven composite samples processed by different pretreatments. Error bars show standard deviations.
A total of 386 Micromonospora-like isolates were purified from HVA, ISP 3, and DNBA plates that had been inoculated with samples processed by the 1.5% phenol or wet-heat pretreatment. Representatives of the isolates showed morphologies typical of the genus Micromonospora, that is, the substrate mycelium bore single spores with smooth, rough, or warty surfaces (data not shown).
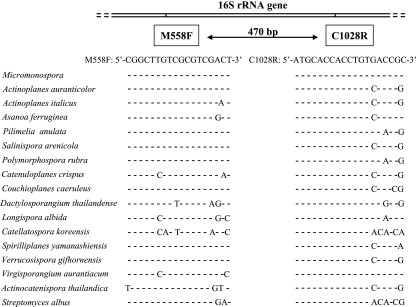
An examination of the aligned 16S rRNA gene sequences of the reference Micromonospora strains and the nontarget marker strains (see Table S1 in the supplemental material) showed that a combination of two regions between positions 558 and 575 and positions 1009 and 1028 [Streptomyces coelicolor A3(2) numbering, GenBank accession number Y00411 (2)] was unique for all reference Micromonospora strains we used (Fig. 2). Sequences of these conserved regions were used to design the genus-specific primers, namely, M558F (5′-CGGCTTGTCGCGTCGACT-3′) and C1028R (5′-ATGCACCACCTGTGACCGC-3′). When this primer pair was tested by PCR at an annealing temperature of 68.5°C, which is 2.5 degrees higher than the defined optimum for them, the expected amplification product of 470 bp was obtained from all reference strains of the genus Micromonospora and no amplification product was obtained from strains of other genera (see Table S1 in the supplemental material). At an annealing temperature lower than 68.5°C, false-positive amplification was observed for some species of the genera Actinoplanes and Asanoa.
FIG. 2.
Representative nucleotide sequence alignments with Micromonospora genus-specific primers M558F and C1028R targeting the 16S rRNA gene and examples of mismatches outside the genus, demonstrating the specificity of the primer pair.
Forty-five representative Micromonospora-like isolates, along with 18 strains that were also isolated in this study but assigned to non-Micromonospora actinomycetes based on their morphologies, were chosen to test the specificity of the PCR primers and conditions. A positive amplification was obtained from 42 of the 45 (93.3%) Micromonospora-like isolates, while no amplification was obtained from the 18 non-Micromonospora isolates. Using this pair of primers and a nested PCR technique, we also amplified community DNA from the soil samples with positive results, which showed its use in detecting Micromonospora in environmental samples.
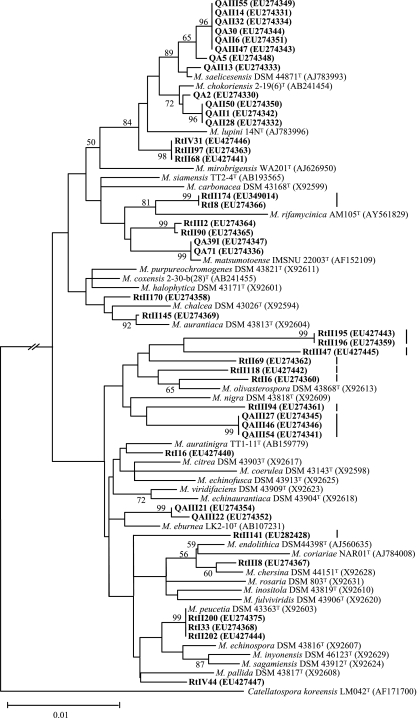
Almost-complete 16S rRNA gene sequences (>1,380 nucleotides) were obtained for all the PCR-positive and -negative isolates. Comparisons of these sequences against the GenBank database by using the NCBI BLASTN program verified that all of the PCR-positive isolates belonged to the genus Micromonospora and the negative ones did not, demonstrating perfect accuracy for Micromonospora recognition by specific PCR, but there was a misidentification rate of 6.7% (3 of 45) in our study on the basis of morphological properties. The three Micromonospora-like but PCR-negative isolates belonged to Polymorphospora, Pseudonocardiaceae, and Nonomuraea, respectively. The representative Micromonospora isolates shared >98% 16S rRNA sequence similarities with the valid species of this genus and formed diverse phyletic lineages interspersed among the Micromonospora 16S rRNA gene tree (Fig. 3). Using a 16S rRNA sequence similarity level of 99%, the isolates grouped into 16 phylotypes, of which 9 (56.3%) have not been previously described based on sharing <99% sequence similarity with any of the type strains in the genus. The level of 99% for phylotype definition was chosen based on statistical evidence for the relationship of 16S rRNA sequence similarity to DNA-DNA reassociation in actinomycetes, where a sequence similarity of ≥99% minimized DNA-DNA reassociation values of <70% (25). According to this evidence, the undescribed phylotypes (Fig. 3) are likely to represent novel Micromonospora species.
FIG. 3.
Neighbor-joining tree based on 16S rRNA gene sequences of 42 representative Micromonospora isolates and 33 type strains of the genus showing phylogenetic relationships between the isolates and Micromonospora species. The isolates are shown in boldface, and GenBank accession numbers are given in parentheses. Undescribed phylotypes are marked with vertical lines on the right of the sequence items. Percentage bootstrap values based on 1,000 resampled data sets are shown at the nodes; only values above 50% are given. The scale bar indicates 0.01 nucleotide substitution per nucleotide position.
Based on the results presented above, we believe that the selective isolation procedures and the rapid identification technique developed in this study will provide facile means for discovering various Micromonospora strains for both industrial exploitation and ecological research.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences determined for strains in this study have been deposited in the GenBank database under the accession numbers EU274329 to EU274377, EU427440 to EU427446, EU282428, and EU349014.
Supplementary Material
Acknowledgments
We are grateful to Kui Hong of Institute of Tropical Biosciences and Biotechnology, CATAS, Haikou, China, for providing the mangrove soil samples. We also thank CGMCC (China General Microbiological Culture Collection Center) and JCM (Japan Collection of Microorganisms) for providing reference strains.
This publication was supported by the Hi-Tech Research and Development Program of China (grant no. 2007AA09Z420).
Footnotes
Published ahead of print on 11 July 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Antal, N., H. P. Fiedler, E. Stackebrandt, W. Beil, K. Stroch, and A. Zeeck. 2005. Retymicin, galtamycin B, saquayamycin Z and ribofuranosyllumichrome, novel secondary metabolites from Micromonospora sp. Tu 6368. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. (Tokyo) 58:95-102. [DOI] [PubMed] [Google Scholar]
- 2.Baylis, H. A., and M. J. Bibb. 1987. The nucleotide sequence of a 16S rRNA gene from Streptomyces coelicolor A3(2). Nucleic Acids Res. 15:7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berdy, J. 2005. Bioactive microbial metabolites. J. Antibiot. (Tokyo) 58:1-26. [DOI] [PubMed] [Google Scholar]
- 4.Colquhoun, J. A., J. Mexson, M. Goodfellow, A. C. Ward, K. Horikoshi, and A. T. Bull. 1998. Novel rhodococci and other mycolate actinomycetes from the deep sea. Antonie van Leeuwenhoek 74:27-40. [DOI] [PubMed] [Google Scholar]
- 5.Gause, G. F., T. P. Preobrazhenskaya, G. V. Sveshnikova, L. P. Terekhova, and T. S. Maksimova. 1983. A guide for determination of actinomycetes. Nauka, Moscow, Russia. (In Russian.)
- 6.Gontang, E. A., W. Fenical, and P. R. Jensen. 2007. Phylogenetic diversity of gram-positive bacteria cultured from marine sediments. Appl. Environ. Microbiol. 73:3272-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez-Lugo, M. T., G. M. Woldemichael, M. P. Singh, P. A. Suarez, W. M. Maiese, G. Montenegro, and B. N. Timmermann. 2005. Isolation of three new naturally occurring compounds from the culture of Micromonospora sp. P1068. Nat. Prod. Res. 19:645-652. [DOI] [PubMed] [Google Scholar]
- 8.Hayakawa, M., and H. Nonomura. 1987. Humic acid vitamin agar, a new medium for the selective isolation of soil actinomycetes. J. Ferment. Technol. 65:501-509. [Google Scholar]
- 9.Hayakawa, M., Sadakata, T. Kajiura, and H. Nonomura. 1991. New methods for the highly selective isolation of Micromonospora and Microbispora from soil. J. Ferment. Bioeng. 72:320-326. [Google Scholar]
- 10.Hernandez, L. M., J. A. Blanco, J. P. Baz, J. L. Puentes, F. R. Millan, F. E. Vazquez, R. I. Fernandez-Chimeno, and D. G. Gravalos. 2000. 4′-N-Methyl-5′-hydroxystaurosporine and 5′-hydroxystaurosporine, new indolocarbazole alkaloids from a marine Micromonospora sp. strain. J. Antibiot. (Tokyo) 53:895-902. [DOI] [PubMed] [Google Scholar]
- 11.Houge-Frydrych, C. S., S. A. Readshaw, and D. J. Bell. 2000. SB-219383, a novel tyrosyl tRNA synthetase inhibitor from a Micromonospora sp. II. Structure determination. J. Antibiot. (Tokyo) 53:351-356. [DOI] [PubMed] [Google Scholar]
- 12.Janssen, P. H., P. S. Yates, B. E. Grinton, P. M. Taylor, and M. Sait. 2002. Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl. Environ. Microbiol. 68:2391-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawato, M., and R. Shinobu. 1959. On Streptomyces herbaricolor sp. nov., supplement: a single technique for microscopical observation. Mem. Osaka Univ. Lib. Arts Educ. B 8:114-119. [Google Scholar]
- 14.Kroppenstedt, R. M., S. Mayilraj, J. M. Wink, W. Kallow, P. Schumann, C. Secondini, and E. Stackebrandt. 2005. Eight new species of the genus Micromonospora, Micromonospora citrea sp. nov., Micromonospora echinaurantiaca sp. nov., Micromonospora echinofusca sp. nov. Micromonospora fulviviridis sp. nov., Micromonospora inyonensis sp. nov., Micromonospora peucetia sp. nov., Micromonospora sagamiensis sp. nov., and Micromonospora viridifaciens sp. nov. Syst. Appl. Microbiol. 28:328-339. [DOI] [PubMed] [Google Scholar]
- 15.Kuester, E., and S. T. Williams. 1964. Selection of media for isolation of Streptomycetes. Nature 202:928-929. [DOI] [PubMed] [Google Scholar]
- 16.Kutzner, H. J. 1986. The family Streptomycetaceae, p. 2028-2090. In M. P. Starr, H. Stolp, H. G. Trüper, A. Balows, and H. G. Schlegel (ed.), The prokaryotes, a handbook on habitats, isolation, and identification of bacteria, vol. 2. Springer-Verlag, New York, NY. [Google Scholar]
- 17.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, England.
- 18.Laurent, F. J., F. Provost, and P. Boiron. 1999. Rapid identification of clinically relevant Nocardia species to genus level by 16S rRNA gene PCR. J. Clin. Microbiol. 37:99-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldonado, L. A., J. E. Stach, W. Pathom-aree, A. C. Ward, A. T. Bull, and M. Goodfellow. 2005. Diversity of cultivable actinobacteria in geographically widespread marine sediments. Antonie van Leeuwenhoek 87:11-18. [DOI] [PubMed] [Google Scholar]
- 20.Mordarska, H., M. Mordarski, and M. Goodfellow. 1972. Chemotaxonomic characters and classification of some nocardioform bacteria. J. Gen. Microbiol. 71:77-86. [DOI] [PubMed] [Google Scholar]
- 21.Moron, R., I. Gonzalez, and O. Genilloud. 1999. New genus-specific primers for the PCR identification of members of the genera Pseudonocardia and Saccharopolyspora. Int. J. Syst. Bacteriol. 49:149-162. [DOI] [PubMed] [Google Scholar]
- 22.Rintala, H., A. Nevalainen, E. Ronka, and M. Suutari. 2001. PCR primers targeting the 16S rRNA gene for the specific detection of streptomycetes. Mol. Cell. Probes 15:337-347. [DOI] [PubMed] [Google Scholar]
- 23.Rusnak, K., J. Troyanovich, R. Mierzwa, M. Chu, M. Patel, and M. Weinstein. 2001. An antibiotic with activity against gram-positive bacteria from the gentamicin-producing strain of Micromonospora purpurea. Appl. Microbiol. Biotechnol. 56:502-503. [DOI] [PubMed] [Google Scholar]
- 24.Salazar, O., I. Gonzalez, and O. Genilloud. 2002. New genus-specific primers for the PCR identification of novel isolates of the genera Nocardiopsis and Saccharothrix. Int. J. Syst. Evol. Microbiol. 52:1411-1421. [DOI] [PubMed] [Google Scholar]
- 25.Stach, J. E., L. A. Maldonado, D. G. Masson, A. C. Ward, M. Goodfellow, and A. T. Bull. 2003. Statistical approaches for estimating actinobacterial diversity in marine sediments. Appl. Environ. Microbiol. 69:6189-6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki, S., T. Okuda, and S. Komatsubara. 1999. Selective isolation and distribution of Sporichthya strains in soil. Appl. Environ. Microbiol. 65:1930-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi, Y., and S. Omura. 2003. Isolation of new actinomycete strains for the screening of new bioactive compounds. J. Gen. Appl. Microbiol. 49:141-154. [DOI] [PubMed] [Google Scholar]
- 28.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 29.Tan, G. Y., A. C. Ward, and M. Goodfellow. 2006. Exploration of Amycolatopsis diversity in soil using genus-specific primers and novel selective media. Syst. Appl. Microbiol. 29:557-569. [DOI] [PubMed] [Google Scholar]
- 30.Zhao, H., Y. Kassama, M. Young, D. B. Kell, and R. Goodacre. 2004. Differentiation of Micromonospora isolates from a coastal sediment in Wales on the basis of Fourier transform infrared spectroscopy, 16S rRNA sequence analysis, and the amplified fragment length polymorphism technique. Appl. Environ. Microbiol. 70:6619-6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhi, X. Y., S. K. Tang, W. J. Li, L. H. Xu, and C. L. Jiang. 2006. New genus-specific primers for the PCR identification of novel isolates of the genus Streptomonospora. FEMS Microbiol. Lett. 263:48-53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.