Abstract
Legionella pneumophila type II secretion mutants showed reduced survival in both tap water at 4 to 17°C and aquatic amoebae at 22 to 25°C. Wild-type supernatants stimulated the growth of these mutants, indicating that secreted factors promote low-temperature survival. There was a correlation between low-temperature survival and secretion function when 12 additional Legionella species were examined.
Legionella pneumophila is widespread in natural and man-made water systems (8, 21, 29, 39, 41, 47, 53, 64). In these habitats, L. pneumophila exists planktonically, within protozoa, and in biofilms (14, 35, 36, 41, 45). The ubiquity of L. pneumophila is also a result of the organism's ability to survive at many temperatures, including ones as low as 4°C (21, 29, 62, 64). L. pneumophila is an important pathogen of humans, with the inhalation of contaminated water droplets originating from aerosol-generating devices resulting in Legionnaires' disease (16). Given the manner in which infection occurs, it is important to better understand how legionellae survive in water, in protozoa, and at low temperatures. Recently, we found that L. pneumophila type II protein secretion is critical for growth in rich broth or agar at 12 to 25°C but not in medium at 30 to 37°C (56). Operative in many gram-negatives (9), type II secretion is a multistep process in which proteins are translocated across the inner membrane in a Sec- or Tat-dependent manner, recognized in the periplasm, and then delivered to the T2S apparatus, whereupon a pilus-like structure “pushes” proteins through a dedicated outer membrane pore or secretin (28).
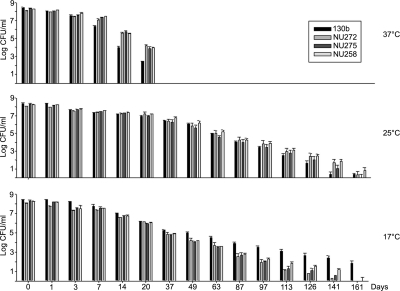
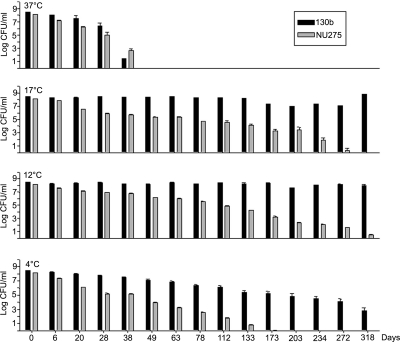
To investigate the connection between type II secretion and low-temperature survival under conditions that more closely mimic natural habitats, we compared wild-type serogroup 1 strain 130b (Table 1) and its type II secretion mutants for persistence in tap water incubated at 37°C, 25°C, and 17°C. We used three mutants: NU258, containing a mutation in the genes encoding the type II outer membrane secretin (lspD) and the inner membrane ATPase (lspE); NU275, containing a mutation in the gene for the inner membrane platform protein (lspF); and NU272 mutated in the gene encoding the pseudopilin peptidase (pilD) (51). Tap water was obtained from laboratory sinks and filter sterilized. Following growth at 37°C in buffered yeast extract (BYE) broth to late log phase (56), wild types and mutants were inoculated into flasks containing 50 ml of the tap water, and then the cultures were incubated with shaking. As with other wild-type L. pneumophila (30, 41, 42, 54, 57), 130b persisted in low-temperature tap water for extended times (Fig. 1). Also similar to previous work (27), the recovery of CFU was maintained for a longer period at low temperatures below 37°C. But across the 17 to 37°C range, the secretion mutants behaved differently than their parent (Fig. 1). At 37°C, the mutants displayed a greater recoverability than 130b between days 7 and 20 (P < 0.05). In a similar vein, at 25°C, the mutants were recovered more than the wild type was between days 126 and 141, although the differences were not statistically significant. But at 17°C, the situation reversed: between days 49 and 161, there was less recovery of the mutants (P < 0.05). These data imply that type II secretion mutants have reduced survival in tap water at 17°C. That independently derived mutants, inactivated for three different genes, representing three different transcriptional units, including two that are solely dedicated to type II secretion (51), behaved similarly indicated that this survival defect was due to the loss of type II secretion function versus second-site mutations. To confirm that type II mutants have reduced survival in water at low temperatures, we retested the lspF mutant in a new water sample incubated at 37°C, 17°C, 12°C, and 4°C. This experiment was started 20 months after the first in order to see if the survival differences were peculiar to certain water samples or not. Again, 130b persisted for long periods of time, especially at temperatures below 37°C (Fig. 2). Indicating that the tap water had changed appreciably, the recovery of 130b was fully maintained at 17°C for 318 days rather than gradually declining over 161 days. Strain 130b was also fully maintained at 12°C, while at 4°C a gradual decline was seen. We suspect that these changes in survival were a manifestation of changing biocides, but details on the treatments used in our local environment were not available to us. Regardless of the extended recovery of 130b, the secretion mutant distinguished itself in the same way that it had in the first experiment. While briefly showing greater survival at 37°C (P of <0.05 on day 38), the mutant exhibited markedly reduced numbers at 17°C and below (P of <0.05 from day 20 on) (Fig. 2). The mutant phenotype increased in magnitude as temperatures went from 17°C to 4°C. That the large declines in mutant recoverability at low temperatures were due to corresponding losses in viability was verified by examining samples with Live/Dead staining (Molecular Probes, Eugene, OR). These data indicate that an intact type II secretion system is needed for the optimal survival of L. pneumophila in environmental waters at low temperatures.
TABLE 1.
Legionella species and their low-temperature growtha
| Species | Strainb | Source (reference) | 17°C growthc | Proteased |
|---|---|---|---|---|
| L. pneumophila | BAA-74 | Clinical (15, 52) | +++ | + |
| L. anisa | 35292 | Environmentald (22) | +++ | + |
| L. brunensis | 43878 | Environmental (63) | − | − |
| L. cincinnatiensis | 43753 | Clinical (58) | ++ | + |
| L. erythra | 35303 | Environmental (7) | ++ | + |
| L. feeleii | 35072 | Clinical (26) | ++ | + |
| L. hackeliae | 35250 | Clinical (7) | − | − |
| L. londiniensis | 49505 | Environmental (12) | − | − |
| L. longbeachae | 33462 | Clinical (37) | +++ | + |
| L. micdadei | Stanford-R | Clinical (48) | − | − |
| 33218 | Clinical (25) | − | − | |
| 31B | Clinical (48) | + | − | |
| L. moravica | 43877 | Environmental (63) | +++ | + |
| L. oakridgensis | 33761 | Environmentale (40) | − | − |
| L. parisiensis | 35299 | Environmentale (7) | ++ | + |
+, modest growth; ++, good growth; +++, robust growth; −, no growth.
Except for L. micdadei 31B and Stanford-R, the designations refer to ATCC numbers.
The relative bacterial efficiency of plating on BCYE agar at 17°C, as observed on three independent occasions and as exemplified in Fig. 5.
Casein hydrolysis, as observed at 37°C on at least three independent occasions.
FIG. 1.
Survival of wild-type and type II secretion mutant L. pneumophila in tap water at 37°C, 25°C, and 17°C. Wild-type strain 130b, pilD mutant NU272, lspF mutant NU275, and lspDE mutant NU258 were inoculated at comparable levels into sterile tap water, and then the samples were incubated at either 37°C (top), 25°C (middle), or 17°C (bottom). On the indicated days, the numbers of CFU in the samples, presented as means and standard deviations, were determined by plating aliquots on BCYE agar.
FIG. 2.
Survival of wild-type and lspF mutant L. pneumophila at 37°C, 17°C, 12°C, and 4°C. Wild-type strain 130b and lspF mutant NU275 were inoculated at comparable levels into a second sample of sterile tap water and then incubated at the indicated temperatures. At the noted time points, the numbers of CFU in the samples, presented as means and standard deviations, were determined by plating aliquots on BCYE agar.
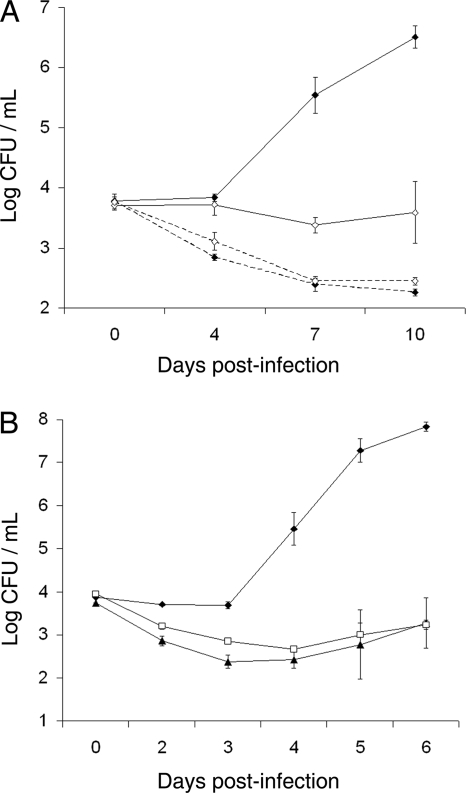
Amoebae are natural hosts for L. pneumophila, and the coincidence of amoebae and legionellae has been verified in low-temperature waters (43, 44). Thus, to determine if the importance of type II secretion at low temperatures is manifest in a mimic of environmental intracellular growth, we compared 130b and its secretion mutants for infection of Acanthamoeba castellanii (ATCC 30234) and Hartmannella vermiformis (ATCC 50237) at 22 to 25°C. Briefly, 104 CFU of bacteria were added to wells containing 105 amoebae, and then at various times, the numbers of bacteria per coculture were determined by plating on buffered charcoal yeast extract (BCYE) agar (38, 50, 51). Upon the infection of acanthamoebae, 130b increased in numbers 103- to 104-fold over a 10-day period (Fig. 3A). In marked contrast, lspF mutant NU275 did not exhibit any evidence of growth, although the parent and the mutant showed like reductions in survival when incubated in medium alone. A similar situation was observed when bacteria infected the hartmannellae (Fig. 3B). Whereas 130b grew ca. 104-fold, pilD mutant NU272 and lspDE mutant NU258 did not increase significantly during the 6-day infection. Amoebal entry assays, done as previously described (24, 60), revealed that 130b, NU275, and NU258 have comparable rates of entry into both A. castellanii and H. vermiformis at 22°C and 37°C (data not shown). Taken together, these data indicate that functional type II secretion is needed for the infection of amoebae at low temperatures and more specifically for intracellular replication.
FIG. 3.
Growth of wild-type and type II secretion mutant L. pneumophila in low-temperature cultures of amoebae. (A) A. castellanii was infected with wild-type strain 130b (⧫) or lspF mutant NU275 (⋄) and then incubated at 22°C. On the indicated days, the numbers of bacteria in the cultures were determined by plating. In parallel, the survival of the parent and the mutant in tissue culture medium alone was assessed (dashed lines). The values presented are the means and standard deviations of the results from four infected wells. On days 4, 7, and 10, the recovery of the lspF mutant was significantly less than that of parental 130b (P < 0.05; Student's t test). Similar results were obtained from four additional experiments, although in some of those experiments, the net growth of wild-type strain 130b was ca. 104-fold versus ca. 103-fold. (B) H. vermiformis was infected with wild-type strain 130b (⧫), pilD mutant NU272 (▴), or lspDE mutant NU258 (□) and then incubated at 25°C. At the indicated times, the numbers of bacteria in the cultures were determined by plating. The values presented are the means and standard deviations of the results from four infected wells and is representative of two independent experiments. On days 2 through 6, the recovery of the mutants was significantly less than that of strain 130b (P < 0.05; Student's t test).
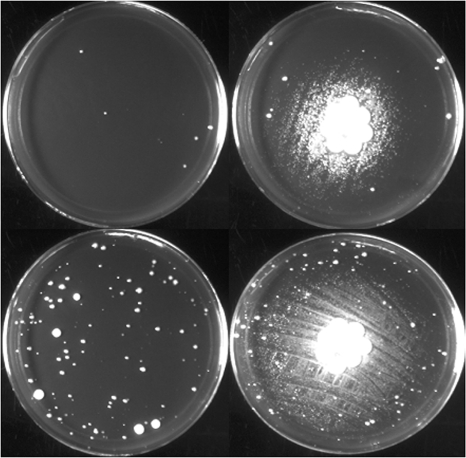
To further investigate the mechanism of low-temperature survival, 130b was grown at 17°C, and then cell-free culture supernatants were tested for their ability to restore low-temperature growth to an lspF mutant. The supernatants greatly stimulated the growth of the mutant when inoculated onto BCYE agar at 17°C (Fig. 4). Exposure to BYE broth that had been incubated at 17°C did not likewise stimulate growth (data not shown). Some mutant colonies arose at low temperatures independently of wild-type stimulation at a lower frequency (Fig. 4); these colonies likely have suppressor mutations. That wild-type supernatants, but not medium controls, rescue mutant growth at low temperatures indicates that a secreted bacterial factor(s) and/or a medium component(s) that is modified by growing legionellae can promote low-temperature survival. Since our type II secretion mutant undergoes a greater degree of leakage than the wild type does when incubated in BYE broth at the low temperature (55), it was not possible for us to reliably use 17°C mutant supernatants in this assay in order to determine whether the growth-stimulatory substance(s) is type II dependent or not.
FIG. 4.
Low-temperature growth stimulation by L. pneumophila supernatants. A total of 105 (top row) and 106 (bottom row) CFU of lspF mutant NU275 was spread onto BCYE agar and then was either overlaid with disks that had been impregnated with 2 ml of cell-free supernatants obtained from 17°C cultures of wild-type strain 130b (right column) or left alone (left column). Images of the growth were obtained after 10 days of incubation at 22°C. Growth stimulation achieved by exposure to wild-type supernatants was also seen in two other independent experiments.
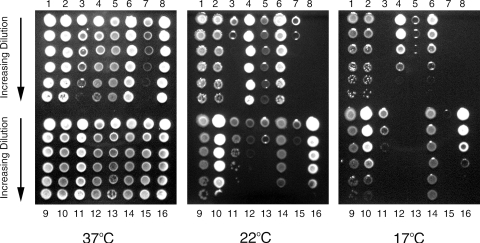
The Legionella genus contains 51 species, besides L. pneumophila, and nearly one-half of the species are implicated in disease (13). To begin to understand the behavior of the other legionellae at low temperatures, we compared 14 strains, representing 12 species (Table 1), for growth on BCYE agar at 37°C, 22°C, and 17°C (Fig. 5). With the exception of L. hackeliae, all strains grew like L. pneumophila did at 37°C. L. anisa, L. longbeachae, and L. moravica also grew comparably to L. pneumophila at the two low temperatures. L. cincinnatiensis, L. erythra, L. feeleii, and L. parisiensis grew like L. pneumophila did at 22°C but showed modest reductions in survival at 17°C. In contrast, L. brunensis, L. hackeliae, L. londiniensis, L. micdadei strains 33218 and Stanford-R, and L. oakridgensis displayed greatly reduced survival at 22°C and a complete inability to grow at 17°C. L. micdadei strain 31B, though clearly showing a reduced capacity to survive at 22 and 17°C, grew better at low temperatures than the other L. micdadei strains. As summarized in Table 1, these data indicate that survival and growth at low temperatures are common but not universal among species of Legionella. The finding that multiple species of Legionella were able to grow on BCYE agar at low temperatures is not surprising, since non-pneumophila legionellae have been detected in many waters, including low-temperature ones (29, 64). But the observation that several species of Legionella were unable to grow or grew very poorly at 22°C and 17°C was unanticipated. Although some studies have suggested that the makeup of the Legionella population is influenced by temperature in warm waters (36, 53, 61), there was no indication in the literature that would have predicted the inability of certain legionellae to survive at low temperatures. Thus, our data indicate, for the first time, that only a subset of Legionella species flourish in cold water systems.
FIG. 5.
Low-temperature growth of different Legionella species. Tenfold serial dilutions of suspensions of L. pneumophila 130b (lanes 1 and 9), L. anisa (lane 2), L. brunensis (lane 3), L. cincinnatiensis (lane 4), L. erythra (lane 5), L. feeleii (lane 6), L. hackeliae (lane 7), L. londiniensis (lane 8), L. longbeachae (lane 10), L. micdadei 31B (lane 11), L. micdadei 33218 (lane 12), L. micdadei Stanford-R (lane 13), L. moravica (lane 14), L. oakridgensis (lane 15), and L. parisiensis (lane 16) were spotted, in the indicated order, onto BCYE agar and then incubated at either 37°C (left), 22°C (center), or 17°C (right). Images of bacterial growth were then obtained after 3 days for the 37°C plates, 13 days for the 22°C plates, or 15 days for the 17°C plates. Because of the reddish pigmentation exhibited by L. erythra (7), the extent of growth in lane 5, center and right, is not fully apparent in the images. These results are representative of two (22°C) or three (37°C, 17°C) independent experiments.
When these various species were grown on agar containing casein, as previously described (23, 51), we observed an intriguing correlation between strong growth at low temperatures and protease activity (Table 1). Whereas species that grew relatively well at 17°C had casein-degrading activity, L. brunensis, L. hackeliae, L. londiniensis, L. micdadei, and L. oakridgensis lacked it. Since caseinolytic activity is indicative of type II secretion in L. pneumophila (23, 51), we hypothesize that those Legionella species that do not grow well at low temperatures lack aspects of type II secretion. Because an L. pneumophila proA mutant specifically lacking casein degradation grows normally at room temperature (56), we suspect that the inability of some legionellae to grow at low temperatures is not simply due to a lack of caseinolytic activity. In support of these hypotheses, past studies have found L. micdadei strains, including the ones used here as well as others, to also lack type II-dependent phosphatase, hemolytic, and lipolytic activities (5, 17, 18, 49, 51). Since L. micdadei and all other Legionella species tested thus far contain lsp genes (51), the deficiencies in low-temperature growth and secreted activity exhibited by some Legionella species are likely due to a lack of expression of the type II secretion apparatus or the absence of (expression of) individual exoproteins.
In the simplest scenario, type II-dependent effectors facilitate extra- and intracellular survival by allowing the bacterium to acquire nutrients and/or combat harsh conditions, such as oxidative stress, biocides, or bactericidal/static agents made by other microbes. That the role of Lsp in environmental mimics is most manifest at lower temperatures may be because nutrients are less available or toxic factors are more prevalent in that situation. For example, dissolved oxygen and thus the potential for oxidative stress increases with decreasing temperature (1). In the absence of secreted effectors, such as would occur for an lsp mutant, increased cell leakage and lysis may ultimately occur. Given what is known of the type II secretome, there are many candidate effectors. For example, when 130b is grown in BYE broth at 37°C, the secretome encompasses more than 25 proteins, including many degradative enzymes as well as proteins with no similarity to known proteins (2-4, 6, 10, 11, 19, 20, 23, 31, 49-51). Also, 2D polyacrylamide gel electrophoresis analysis of supernatants produced during growth at 12 to 17°C revealed other exoproteins that are more pronounced at low temperatures (55). Although more work is needed to identify the critical secreted factors, the new data presented here significantly increase our appreciation for the role that type II secretion plays in Legionella ecology. In the past, we and others had shown the importance of Lsp for the infection of host cells and lung tissue at 35 to 37°C (23, 31, 46, 49, 51), while others suggested a role in biofilms at 30°C (34). Thus, we can now state that Lsp is important in the full range of Legionella niches, from planktonic and intracellular aquatic niches to the extra- and intracellular niches in the human host and from low-temperature conditions to high-temperature situations. Current findings also represent the first identification of Legionella genes that are necessary for optimal survival in low-temperature aquatic habitats and as such may constitute potential targets for minimizing the risk posed by legionellae in water systems. Finally, based on these latest findings in Legionella, it is plausible that the type II secretion systems of other bacteria, including pathogenic species of Aeromonas, Burkholderia, Erwinia, Pseudomonas, Vibrio, and Xanthomonas, are especially critical for persistence in low-temperature water habitats.
Acknowledgments
We thank past and present members of the Cianciotto laboratory for their assistance and helpful comments.
This work was supported by NIH grant AI43987 awarded to N.P.C.
Footnotes
Published ahead of print on 11 July 2008.
REFERENCES
- 1.Abrahams, M. V., M. Mangel, and K. Hedges. 2007. Predator-prey interactions and changing environments: who benefits? Philos. Trans. R. Soc. Lond. B 362:2095-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aragon, V., S. Kurtz, and N. P. Cianciotto. 2001. Legionella pneumophila major acid phosphatase and its role in intracellular infection. Infect. Immun. 69:177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aragon, V., S. Kurtz, A. Flieger, B. Neumeister, and N. P. Cianciotto. 2000. Secreted enzymatic activities of wild-type and pilD-deficient Legionella pneumophila. Infect. Immun. 68:1855-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aragon, V., O. Rossier, and N. P. Cianciotto. 2002. Legionella pneumophila genes that encode lipase and phospholipase C activities. Microbiology 148:2223-2231. [DOI] [PubMed] [Google Scholar]
- 5.Baine, W. B. 1985. Cytolytic and phospholipase C activity in Legionella species. J. Gen. Microbiol. 131:1383-1391. [DOI] [PubMed] [Google Scholar]
- 6.Banerji, S., M. Bewersdorff, B. Hermes, N. P. Cianciotto, and A. Flieger. 2005. Characterization of the major secreted zinc metalloprotease-dependent glycerophospholipid:cholesterol acyltransferase, PlaC, of Legionella pneumophila. Infect. Immun. 73:2899-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner, D. J., A. G. Steigerwalt, G. W. Gorman, H. W. Wilkinson, W. F. Bibb, M. Hackel, R. L. Tyndall, J. Campbell, J. C. Feeley, W. L. Thacker, P. Skaliy, W. T. Martin, B. J. Brake, B. S. Fields, H. V. McEachern, and L. K. Corcoran. 1985. Ten new species of Legionella. Int. J. Syst. Bacteriol. 35:50-59. [Google Scholar]
- 8.Carvalho, F. R., R. F. Vazoller, A. S. Foronda, and V. H. Pellizari. 2007. Phylogenetic study of Legionella species in pristine and polluted aquatic samples from a tropical Atlantic forest ecosystem. Curr. Microbiol. 55:288-293. [DOI] [PubMed] [Google Scholar]
- 9.Cianciotto, N. P. 2005. Type II secretion: a protein secretion system for all seasons. Trends Microbiol. 13:581-588. [DOI] [PubMed] [Google Scholar]
- 10.DebRoy, S., V. Aragon, S. Kurtz, and N. P. Cianciotto. 2006. Legionella pneumophila Mip, a surface-exposed peptidylproline cis-trans-isomerase, promotes the presence of phospholipase C-like activity in culture supernatants. Infect. Immun. 74:5152-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DebRoy, S., J. Dao, M. Soderberg, O. Rossier, and N. P. Cianciotto. 2006. Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc. Natl. Acad. Sci. USA 103:19146-19151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennis, P. J., D. J. Brenner, W. L. Thacker, R. Wait, G. Vesey, A. G. Steigerwalt, and R. F. Benson. 1993. Five new Legionella species isolated from water. Int. J. Syst. Bacteriol. 43:329-337. [DOI] [PubMed] [Google Scholar]
- 13.Diederen, B. M. 2008. Legionella spp. and Legionnaires' disease. J. Infect. 56:1-12. [DOI] [PubMed] [Google Scholar]
- 14.Donlan, R. M., T. Forster, R. Murga, E. Brown, C. Lucas, J. Carpenter, and B. Fields. 2005. Legionella pneumophila associated with the protozoan Hartmannella vermiformis in a model multi-species biofilm has reduced susceptibility to disinfectants. Biofouling 21:1-7. [DOI] [PubMed] [Google Scholar]
- 15.Engleberg, N. C., D. J. Drutz, and B. I. Eisenstein. 1984. Cloning and expression of Legionella pneumophila antigens in Escherichia coli. Infect. Immun. 44:222-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields, B. S., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flieger, A., S. Gong, M. Faigle, M. Deeg, P. Bartmann, and B. Neumeister. 2000. Novel phospholipase A activity secreted by Legionella species. J. Bacteriol. 182:1321-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flieger, A., S. Gong, M. Faigle, H. Northoff, and B. Neumeister. 2001. In vitro secretion kinetics of proteins from Legionella pneumophila in comparison to proteins from non-pneumophila species. Microbiology 147:3127-3134. [DOI] [PubMed] [Google Scholar]
- 19.Flieger, A., S. Gong, M. Faigle, S. Stevanovic, N. P. Cianciotto, and B. Neumeister. 2001. Novel lysophospholipase A secreted by Legionella pneumophila. J. Bacteriol. 183:2121-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flieger, A., B. Neumeister, and N. P. Cianciotto. 2002. Characterization of the gene encoding the major secreted lysophospholipase A of Legionella pneumophila and its role in detoxification of lysophosphatidylcholine. Infect. Immun. 70:6094-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fliermans, C. B., W. B. Cherry, L. H. Orrison, S. J. Smith, D. L. Tison, and D. H. Pope. 1981. Ecological distribution of Legionella pneumophila. Appl. Environ. Microbiol. 41:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorman, G. W., J. C. Feeley, A. Steigerwalt, P. H. Edelstein, C. W. Moss, and D. J. Brenner. 1985. Legionella anisa: a new species of Legionella isolated from potable waters and a cooling tower. Appl. Environ. Microbiol. 49:305-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hales, L. M., and H. A. Shuman. 1999. Legionella pneumophila contains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect. Immun. 67:3662-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harb, O. S., C. Venkataraman, B. J. Haack, L.-Y. Gao, and Y. A. Kwaik. 1998. Heterogeneity in the attachment and uptake mechanisms of the Legionnaires' disease bacterium, Legionella pneumophila, by protozoan hosts. Appl. Environ. Microbiol. 64:126-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hebert, G. A., C. W. Moss, L. K. McDougal, F. M. Bozeman, R. M. McKinney, and D. J. Brenner. 1980. The rickettsia-like organisms TATLOCK (1943) and HEBA (1959): bacteria phenotypically similar to but genetically distinct from Legionella pneumophila and the WIGA bacterium. Ann. Intern. Med. 92:45-52. [DOI] [PubMed] [Google Scholar]
- 26.Herwaldt, L. A., G. W. Gorman, T. McGrath, S. Toma, B. Brake, A. W. Hightower, J. Jones, A. L. Reingold, P. A. Boxer, P. W. Tang, C. W. Moss, H. Wilkinson, D. J. Brenner, A. G. Steigerwalt, and C. V. Broome. 1984. A new Legionella species, Legionella feeleii species nova, causes Pontiac fever in an automobile plant. Ann. Intern. Med. 100:333-338. [DOI] [PubMed] [Google Scholar]
- 27.Hussong, D., R. R. Colwell, M. O'Brien, E. Weiss, A. D. Pearson, R. M. Weiner, and W. D. Burge. 1987. Viable Legionella pneumophila not detectable by culture on agar media. Bio/Technology 5:947-950. [Google Scholar]
- 28.Johnson, T. L., J. Abendroth, W. G. Hol, and M. Sandkvist. 2006. Type II secretion: from structure to function. FEMS Microbiol. Lett. 255:175-186. [DOI] [PubMed] [Google Scholar]
- 29.Joly, J. R., M. Boissinot, J. Duchaine, M. Duval, J. Rafrafi, D. Ramsay, and R. Letarte. 1984. Ecological distribution of Legionellaceae in the Quebec City area. Can. J. Microbiol. 30:63-67. [DOI] [PubMed] [Google Scholar]
- 30.Kusnetsov, J. M., P. J. Keskitalo, H. E. Ahonen, A. I. Tulkki, I. T. Miettinen, and P. J. Martikanen. 1994. Growth of Legionella and other heterotrophic bacteria in a circulating cooling water system exposed to ultraviolet irradiation. J. Appl. Bacteriol. 77:461-466. [DOI] [PubMed] [Google Scholar]
- 31.Liles, M. R., P. H. Edelstein, and N. P. Cianciotto. 1999. The prepilin peptidase is required for protein secretion by and the virulence of the intracellular pathogen Legionella pneumophila. Mol. Microbiol. 31:959-970. [DOI] [PubMed] [Google Scholar]
- 32.Lo Presti, F., S. Riffard, S. Jarraud, F. Le Gallou, H. Richet, F. Vandenesch, and J. Etienne. 2000. Isolation of Legionella oakridgensis from two patients with pleural effusion living in the same geographical area. J. Clin. Microbiol. 38:3128-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo Presti, F., S. Riffard, F. Vandenesch, M. Reyrolle, E. Ronco, P. Ichai, and J. Etienne. 1997. The first clinical isolate of Legionella parisiensis, from a liver transplant patient with pneumonia. J. Clin. Microbiol. 35:1706-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucas, C. E., E. Brown, and B. S. Fields. 2006. Type IV pili and type II secretion play a limited role in Legionella pneumophila biofilm colonization and retention. Microbiology 152:3569-3573. [DOI] [PubMed] [Google Scholar]
- 35.Mampel, J., T. Spirig, S. S. Weber, J. A. Haagensen, S. Molin, and H. Hilbi. 2006. Planktonic replication is essential for biofilm formation by Legionella pneumophila in a complex medium under static and dynamic flow conditions. Appl. Environ. Microbiol. 72:2885-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marrao, G., A. Verissimo, R. G. Bowker, and M. S. daCosta. 1993. Biofilms as major sources of Legionella spp. in hydrothermal areas and their dispersion into stream water. FEMS Microbiol. Ecol. 12:25-33. [Google Scholar]
- 37.McKinney, R. M., R. K. Porschen, P. H. Edelstein, M. L. Bissett, P. P. Harris, S. P. Bondell, A. G. Steigerwalt, R. E. Weaver, M. E. Ein, D. S. Lindquist, R. S. Kops, and D. J. Brenner. 1981. Legionella longbeachae species nova, another etiologic agent of human pneumonia. Ann. Intern. Med. 94:739-743. [DOI] [PubMed] [Google Scholar]
- 38.Moffat, J. F., P. H. Edelstein, D. P. Regula, Jr., J. D. Cirillo, and L. S. Tompkins. 1994. Effects of an isogenic Zn-metalloprotease-deficient mutant of Legionella pneumophila in a guinea-pig pneumonia model. Mol. Microbiol. 12:693-705. [DOI] [PubMed] [Google Scholar]
- 39.Mouchtouri, V., E. Velonakis, A. Tsakalof, C. Kapoula, G. Goutziana, A. Vatopoulos, J. Kremastinou, and C. Hadjichristodoulou. 2007. Risk factors for contamination of hotel water distribution systems by Legionella species. Appl. Environ. Microbiol. 73:1489-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orrison, L. H., W. B. Cherry, R. L. Tyndall, C. B. Fliermans, S. B. Gough, M. A. Lambert, L. K. McDougal, W. F. Bibb, and D. J. Brenner. 1983. Legionella oakridgensis: unusual new species isolated from cooling tower water. Appl. Environ. Microbiol. 45:536-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paszko-Kolva, C., M. Shahamat, and R. R. Colwell. 1993. Effect of temperature on survival of Legionella pneumophila in the aquatic environment. Microb. Releases 2:73-79. [PubMed] [Google Scholar]
- 42.Paszko-Kolva, C., M. Shahamat, and R. R. Colwell. 1992. Long-term survival of Legionella pneumophila serogroup 1 under low-nutrient conditions and associated morphological changes. FEMS Microbiol. Ecol. 102:45-55. [Google Scholar]
- 43.Paszko-Kolva, C., M. Shahamat, H. Yamamoto, T. Sawyer, J. Vives-Rego, and R. R. Colwell. 1991. Survival of Legionella pneumophila in the aquatic environment. Microb. Ecol. 22:75-83. [DOI] [PubMed] [Google Scholar]
- 44.Patterson, W. J., J. Hay, D. V. Seal, and J. C. McLuckie. 1997. Colonization of transplant unit water supplies with Legionella and protozoa: precautions required to reduce the risk of legionellosis. J. Hosp. Infect. 37:7-17. [DOI] [PubMed] [Google Scholar]
- 45.Piao, Z., C. C. Sze, O. Barysheva, K. Iida, and S. Yoshida. 2006. Temperature-regulated formation of mycelial mat-like biofilms by Legionella pneumophila. Appl. Environ. Microbiol. 72:1613-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polesky, A. H., J. T. Ross, S. Falkow, and L. S. Tompkins. 2001. Identification of Legionella pneumophila genes important for infection of amoebas by signature-tagged mutagenesis. Infect. Immun. 69:977-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ragull, S., M. Garcia-Nunez, M. L. Pedro-Botet, N. Sopena, M. Esteve, R. Montenegro, and M. Sabria. 2007. Legionella pneumophila in cooling towers: fluctuations in counts, determination of genetic variability by pulsed-field gel electrophoresis (PFGE), and persistence of PFGE patterns. Appl. Environ. Microbiol. 73:5382-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robey, M., W. O'Connell, and N. P. Cianciotto. 2001. Identification of Legionella pneumophila rcp, a pagP-like gene that confers resistance to cationic antimicrobial peptides and promotes intracellular infection. Infect. Immun. 69:4276-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossier, O., and N. P. Cianciotto. 2001. Type II protein secretion is a subset of the PilD-dependent processes that facilitate intracellular infection by Legionella pneumophila. Infect. Immun. 69:2092-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossier, O., J. Dao, and N. P. Cianciotto. 2008. The type II secretion system of Legionella pneumophila elaborates two aminopeptidases as well as a metalloprotease that contributes to differential infection among protozoan hosts. Appl. Environ. Microbiol. 74:753-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossier, O., S. R. Starkenburg, and N. P. Cianciotto. 2004. Legionella pneumophila type II protein secretion promotes virulence in the A/J mouse model of Legionnaires' disease pneumonia. Infect. Immun. 72:310-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saito, A., R. D. Rolfe, P. H. Edelstein, and S. M. Finegold. 1981. Comparison of liquid growth media for Legionella pneumophila. J. Clin. Microbiol. 14:623-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheehan, K. B., J. M. Henson, and M. J. Ferris. 2005. Legionella species diversity in an acidic biofilm community in Yellowstone National Park. Appl. Environ. Microbiol. 71:507-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skaliy, P., and H. V. McEachern. 1979. Survival of the Legionnaires' disease bacterium in water. Ann. Intern. Med. 90:662-663. [DOI] [PubMed] [Google Scholar]
- 55.Söderberg, M. A., and N. P. Cianciotto. 2008. A Legionella pneumophila peptidyl-prolyl cis-trans isomerase present in culture supernatants is necessary for optimal growth at low temperatures. Appl. Environ. Microbiol. 74:1634-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Söderberg, M. A., O. Rossier, and N. P. Cianciotto. 2004. The type II protein secretion system of Legionella pneumophila promotes growth at low temperatures. J. Bacteriol. 186:3712-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinert, M., L. Emödy, R. Amann, and J. Hacker. 1997. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl. Environ. Microbiol. 63:2047-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thacker, W. L., R. F. Benson, J. L. Staneck, S. R. Vincent, W. R. Mayberry, D. J. Brenner, and H. W. Wilkinson. 1988. Legionella cincinnatiensis sp. nov. isolated from a patient with pneumonia. J. Clin. Microbiol. 26:418-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Mee-Marquet, N., A. S. Domelier, L. Arnault, D. Bloc, P. Laudat, P. Hartemann, and R. Quentin. 2006. Legionella anisa, a possible indicator of water contamination by Legionella pneumophila. J. Clin. Microbiol. 44:56-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venkataraman, C., B. J. Haack, S. Bondada, and Y. Abu Kwaik. 1997. Identification of a Gal/GalNAc lectin in the protozoan Hartmannella vermiformis as a potential receptor for attachment and invasion by the Legionnaires' disease bacterium. J. Exp. Med. 186:537-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verissimo, A., G. Marrao, F. Gomes da Silva, and M. S. Da Costa. 1991. Distribution of Legionella spp. in hydrothermal areas in continental Portugal and the island of Sao Miguel, Azores. Appl. Environ. Microbiol. 57:2921-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wadowsky, R. M., R. Wolford, A. M. McNamara, and R. B. Yee. 1985. Effect of temperature, pH, and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl. Environ. Microbiol. 49:1197-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilkinson, H. W., V. Drasar, W. L. Thacker, R. F. Benson, J. Schindler, B. Potuznikova, W. R. Mayberry, and D. J. Brenner. 1988. Legionella moravica sp. nov. and Legionella brunensis sp. nov. isolated from cooling-tower water. Ann. Inst. Pasteur Microbiol. 139:393-402. [DOI] [PubMed] [Google Scholar]
- 64.Wullings, B. A., and D. van der Kooij. 2006. Occurrence and genetic diversity of uncultured Legionella spp. in drinking water treated at temperatures below 15°C. Appl. Environ. Microbiol. 72:157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]