Abstract
Autoaggregation of widely dispersed Xylella fastidiosa cells into compact cell masses occurred over a period of hours following 7 to 11 days of growth in microfluidic chambers. Studies involving the use of mutants defective in polarly positioned type I (fimA-negative), type IV (pilB-negative), or both type I and IV (fimA- and pilO-negative) pili revealed the importance and role of pili in the autoaggregation process.
The gram-negative bacterium Xylella fastidiosa is an important plant pathogen in the warmer regions of the Western Hemisphere. The bacteria cause severe diseases in many hosts such as citrus, grape, almond, coffee, and oleander (19). X. fastidiosa is known to persist only in the water-conducting xylem vessels of plants and in the foregut of different classes of sharpshooter insects that serve as vectors as they feed on xylem fluids (19). The mechanism(s) by which the bacteria cause disease expression is not fully understood; however, the most plausible explanation is that, as they colonize xylem vessels, biofilm-like aggregates block the passage of water and nutrients from the roots to the leaves (20, 27, 36). Other studies suggest that toxins produced by X. fastidiosa could also affect the plant host, resulting in disease expression (28).
Bacterial biofilms occur over a wide range of species and on many environmental surfaces (2, 32). For bacteria living in association with plants, biofilms serve as a means of protection against environmental stresses (5), and for vascular plant pathogens, such as X. fastidiosa, biofilm formation likely serves in absorption of nutrients for the bacteria (27). Biofilm formation is a highly regulated process involving cell adhesion, aggregation, and community expansion (32). In X. fastidiosa, the process of cell aggregation is particularly important and yet is poorly understood.
Many gram-negative bacterial species form cell aggregates that contribute to biofilm development (32, 33). Bacterial cell surface adhesins, and in particular many types of fimbriae and pili, function in cell aggregation and are especially important in the initial stages of adherence to surfaces (32). Interactions between bacteria also contribute to aggregation as well as to microcolony development, steps important in initiation of biofilms. Such interactions are controlled by a range of factors, including quorum sensing mediated by N-acyl homoserine lactones in Pseudomonas aeruginosa (7) and Burkholderia cepacia (21); exopolysaccharides (4, 34); plasmid-encoded type IV bundle-forming pili in Escherichia coli (23); and afimbrial Xad, Hxf, and Hec adhesins in Xanthomonas campestris and X. fastidiosa (12, 14, 15, 24).
In this study we describe a process of cell autoaggregation by X. fastidiosa, an event that was initially discovered during the course of assessing temporal and spatial dynamics of biofilm development by this plant pathogen. Here we report that the process is reliant upon the presence of both type I and type IV pili, with each pilus type playing a specific role in the structural dynamics of the resultant aggregates.
Autoaggregation of X. fastidiosa WT cells.
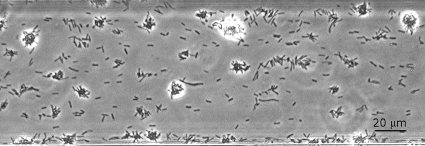
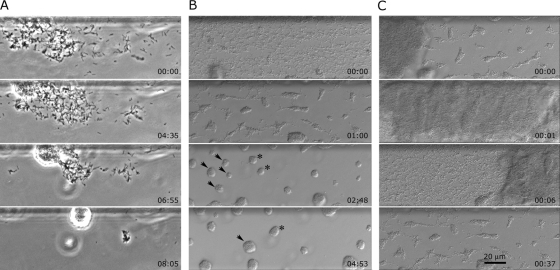
Wild-type (WT) X. fastidiosa (37) cells were grown in microfluidic chambers containing PD2 medium (8). Microfluidic chambers used in this study consisted of two parallel microchannels, each 80 μm wide, 3.7 cm long, and 50 μm deep, and were prepared as previously described (9, 10). Fabrication of these devices was similar to that reported by Meng et al. (26) and in brief consisted of photolithography and deep reactive-ion etching of a silicon wafer followed by replica molding of the wafer surface features with polydimethylsiloxane, which was sandwiched between a glass microscope slide and coverslip. Cells from 4- to 6-day-old bacterial cultures grown on modified periwinkle wilt agar plates (26) were suspended in PD2 broth and introduced into the channels of the chambers. Once the cells attached to the glass surface of the microfluidic channels, medium flow was maintained at 0.2 μl min−1 (equivalent to 1 × 104 μm min−1) for 10 to 12 days. Dynamic aspects of X. fastidiosa cell activities, e.g., type IV pilus twitching motility, cell multiplication, and aggregation, were observed as previously reported using time-lapse video imaging microscopy (9, 10, 26). Within 3 to 5 days many individual cells had formed small star-shaped aggregates comprised of a few to many cells (Fig. 1), most of which were attached to each other at the pilus-bearing poles. By 7 to 11 days, X. fastidiosa cells and small aggregates occupied much of the glass surface within the channels. It is noted that X. fastidiosa is a very slow growing bacterium, with doubling times between 5 and 10 h (13) and as long as 1.9 days (39), thus the long observation times. During this time period the process that we refer to as autoaggregation started and appeared as an accelerated amassing of individual cells and small aggregates. The main characteristic of this process was that dispersed cells merged into compact spherical cell masses (Fig. 2A; see Movie S1 in the supplemental material [all supplemental movies are also present at http://www.nysaes.cornell.edu/pp/faculty/hoch/movies/) over relatively short periods of time, typically 3 to 10 h. Cells continued to be integrated into the developing aggregates until most cells in the region were subsumed by the developing aggregates (Fig. 2A). In general, multiple aggregates formed simultaneously, and these individual aggregates merged over the course of a few hours (Fig. 2B; see Movie S2 in the supplemental material). On occasion, large cell masses broke loose from the channel and were carried downstream, disrupting previously formed aggregates and dispersing the individual cells as they passed (Fig. 2C; see Movie S3 in the supplemental material). The dispersed cells reformed into aggregates within a few hours (Fig. 2C). This dispersal and reformation of aggregates occurred repeatedly. Aggregation, and especially reaggregation, of dispersed X. fastidiosa cells appeared to be related to fimbrial adhesins or possibly to hydrophobic-hydrophilic phenomena that might occur between cells and the medium. Thus, we investigated surface properties of the cells with this in mind.
FIG. 1.
WT Xylella fastidiosa cells forming star-shaped aggregates within a microfluidic chamber. Such aggregates occurred after 3 to 5 days of growth.
FIG. 2.
Time-lapse images of autoaggregation of WT X. fastidiosa cells. (A) Phase-contrast images depicting individual cells being attracted to a developing spherical cell aggregate. (B) Nomarski differential interference contrast (DIC) images illustrating aggregation of cells into distinct aggregates, many of which merge with each other. Individual aggregates, denoted by arrowheads and asterisks, at 02:48 (relative time is indicated as h:min) merged into single aggregates by 04:53. (C) DIC images depicting a large cell aggregate being displaced downstream by medium flow and in the process disrupting smaller aggregates into dispersed cells that quickly reaggregated. Medium flow is left to right. For corresponding movies see the supplemental material.
Influence of pilus type on autoaggregation.
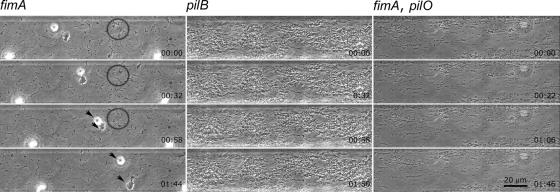
Mutants deficient in genes necessary for production of type I and type IV pili were previously obtained by random mutagenesis using an EZ::TN Tn5 transposome system (16, 25, 26). The parallel-channel design of the microfluidic chambers allowed for simultaneous comparison of different pilus-defective mutants with the WT isolate. A pilY1 mutant (TM14) (10, 25) thought to be defective for a pilus tip protein exhibited aggregation characteristics indistinguishable from those of the WT isolate (Fig. 2; see Movie S1 in the supplemental material). A fimA mutant (6E11) (26) defective for production of type I pili also exhibited autoaggregation events similar to that seen with the WT isolate; however, aggregates formed by the fimA mutant occurred with lower frequency. Time-lapse microscopy revealed that, while individual cells merged to form aggregates, they often dispersed after coming together but eventually formed stable aggregates, albeit somewhat smaller than the WT aggregates (Fig. 3; see Movie S4 in the supplemental material). The process of autoaggregation of the fimA mutant was observed especially in areas of the chamber with significantly reduced flow. This mutant lacks the strong attachment to the substratum conferred by type I pili (9, 10); therefore, the aggregates were easily washed downstream in areas of stronger flow in the chamber.
FIG. 3.
Time-lapse images of aggregation of pilus-defective X. fastidiosa mutants. Cells of the fimA mutant (lacking type I pili) often formed tenuous aggregates (circles, arrowheads). Cells of a pilB mutant (lacking type IV pili) did not form spherical aggregates but instead developed loose lace-like structures. Cells of a double mutant (fimA pilO) defective in both type I and type IV pili similarly did not form aggregates but instead remained dispersed as individual cells. Relative time is indicated as h:min. Medium flow is left to right. For corresponding movies see the supplemental material.
A pilB mutant (1A2) (26) defective for the presence of type IV pili did not form spherical or compact aggregates (Fig. 3; see Movie S5 in the supplemental material). Instead, the cells remained attached to each other (mostly end on end) following cell division, forming lace-like masses often occupying the entire chamber volume. These cell masses were eventually displaced downstream with the medium flow. A fimA pilO double mutant (DM12) (25), defective for both type I and type IV pili, similarly did not exhibit autoaggregation characteristics (Fig. 3; see Movie S6 in the supplemental material). While cells of this mutant multiplied and occupied much of the channel, they remained separated from each other, even in the areas of significantly reduced flow.
Cell surface physical properties.
Considering the clear difference in aggregation behavior between young (a few days old) and older (7 to 11 days old) X. fastidiosa cells, the properties of surface hydrophobicity and charge (zeta potential) were assessed for cells 3 and 8 days old. Hydrophobicity was determined using the technique “bacterial adhesion to hydrocarbons” (29) with some modifications. Briefly, planktonic WT X. fastidiosa cells were harvested and washed twice with potassium-urea-magnesium buffer (29) and then mixed (2:1) with each of three hydrocarbons (n-hexadecane, n-octane, and p-xylene). Affinity of bacterial cells for the organic phase, viz., the percentage of hydrophobicity, was measured as a reduction in optical density of the aqueous phase. Three-day-old cells were significantly (P = 0.0001 for each one of the hydrocarbons) more hydrophobic than 8-day-old cells (Fig. 4). No significant differences in hydrophobicity values between planktonic cells and biofilm cells from the same culture age were noted (data not shown). Higher levels of hydrophobicity among young cells may help in the process of initial cell aggregation, since cells are immersed in a hydrophilic aqueous environment; however, it was anticipated that older cells would have been more hydrophobic as a characteristic contributing to autoaggregation. The increase in hydrophilic properties of older cells may be a consequence of exopolysaccharide production, since these molecules are generally hydrophilic (30, 34).
FIG. 4.
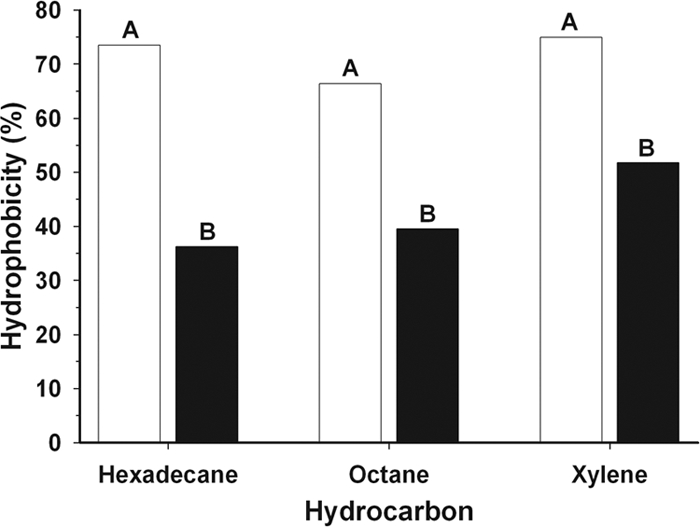
Hydrophobicity of WT X. fastidiosa cells grown for 3 (open bars) and 8 (solid bars) days, assessed by partitioning into three different hydrocarbons. Different letters indicate a significant difference (P = 0.0001) for each hydrocarbon. Data were collected from six independent experiments (three for each time point), which included three or four replicates for each hydrocarbon, and analyzed independently for each hydrocarbon by one-way analysis of variance. Mean separation was performed by Fisher's protected least significant difference test (P = 0.05).
Surface charge, measured as the zeta potential, was determined using a Zetasizer (Malvern Instruments Ltd., Worcestershire, United Kingdom) in a buffer with pH 7.4 and approximate ionic strength of 0.2 M. Three samples from each time point (3 and 8 days) were measured, and no significant (P = 0.11) differences were observed. The average zeta potentials for 3-day-old and 8-day old cells were −16.0 mV and −9.60 mV, respectively. In general, particles with zeta potentials more negative than −30 mV are considered stable. Zeta potential values observed for WT X. fastidiosa cells indicate that these bacterial cells are “nonstable”; thus, the force is not strong enough to prevent cell aggregation.
The autoaggregation phenomenon observed in the present study resembles autoaggregation previously described, for mammalian-associated bacterial pathogens, as flocculation and settling of cells in liquid culture, as clumping of cells, or as formation of spherical bacterial aggregates in tissue culture media (1, 5, 11, 18, 22, 31, 35). All of these descriptions involve a process where cells in suspension rapidly aggregate over a short period of time. The present observations were made at the cell level under dynamic conditions in microfluidic devices, while previous studies were at more gross culture plate or tube level.
The phenomenon of autoaggregation in X. fastidiosa clearly requires the presence of type IV pili, which are also responsible for twitching motility (26). Autoaggregation, as studied in other bacterial species, is triggered by a number of factors depending on the bacterial species and environmental conditions. They include involvement of surface properties such as antigen 43 and type 1 fimbriae in E. coli (17, 31), type IV bundle-forming pili (3), outer membrane proteins in E. coli (38) and Bartonella quintana (40), chemosensory pathways (6) in P. aeruginosa, and temperature in E. coli (3, 31).
As observed in the microfluidic devices, autoaggregation in X. fastidiosa appears to be population density dependent and may explain in part the seemingly “overnight” appearance of Pierce's disease symptoms in infected grapevines. Once the plant is infected, relatively long periods of time (>12 weeks for greenhouse-grown plants) pass before symptoms appear, and then they develop rapidly over a period of a few days. It is possible that widely dispersed X. fastidiosa cells in xylem vessels reach a critical population density, aggregate into large cell masses, and plug the vessels, thus leading to disease symptom expression. In nutrient broth culture, mutants deficient in type IV pili formed thicker biofilms than the WT isolate, while mutants defective in type I pili developed very weak biofilms (25). The results presented in this study suggest that type I and type IV pili are necessary to form stable aggregates that contribute to biofilm formation, which is responsible for obstruction of flow in the xylem vessels of infected plants.
Supplementary Material
Acknowledgments
This work was supported, in part, by grants from the Nanobiotechnology Center (NBTC), an STC program of the National Science Foundation, under agreement no. ECS-9876771 and from the USDA/CSREES administered through the University of California Pierce's Disease Research Grants Program to H.C.H. and T.J.B. This work was also performed, in part, at the Cornell Nanofabrication Facility (a member of the National Nanofabrication Users Network), which is supported by the National Science Foundation under grant ECS-9731293.
Footnotes
Published ahead of print on 18 July 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bieber, D., S. W. Ramer, C.-Y. Wu, W. J. Murray, T. Tobe, R. Fernandez, and G. K. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 2.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 3.Craig, L., M. E. Pique, and J. A. Tainer. 2004. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2:363-378. [DOI] [PubMed] [Google Scholar]
- 4.Crossman, L., and J. M. Dow. 2004. Biofilm formation and dispersal in Xanthomonas campestris. Microbes Infect. 6:623-629. [DOI] [PubMed] [Google Scholar]
- 5.Danhorn, T., and C. Fuqua. 2007. Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 61:401-422. [DOI] [PubMed] [Google Scholar]
- 6.D'Argenio, D. A., M. W. Calfee, P. B. Rainey, and E. C. Pesci. 2002. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J. Bacteriol. 184:6481-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 8.Davis, M. J., W. J. French, and N. W. Schaad. 1981. Axenic culture of the bacteria associated with phony disease of peach and plum leaf scald. Curr. Microbiol. 6:309-314. [Google Scholar]
- 9.De La Fuente, L., E. Montanes, Y. Meng, Y. Li, T. J. Burr, H. C. Hoch, and M. Wu. 2007. Assessing adhesion forces of type I and type IV pili of Xylella fastidiosa using a microfluidic flow chamber. Appl. Environ. Microbiol. 73:2690-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De La Fuente, L., T. J. Burr, and H. C. Hoch. 2007. Mutations in type I and type IV pilus biosynthetic genes affect twitching motility rates in Xylella fastidiosa. J. Bacteriol. 189:7507-7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Re, B., A. Busetto, G. Vignola, B. Sgorbati, and D. Palenzona. 1998. Autoaggregation and adhesion ability in a Bifidobacterium suis strain. Lett. Appl. Microbiol. 27:307-310. [PubMed] [Google Scholar]
- 12.Dow, J. M., L. Crossman, K. Findlay, Y.-Q. He, J. X. Feng, and J. L. Tang. 2003. Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. USA 100:10995-11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feil, H., and A. H. Purcell. 2001. Temperature-dependent growth and survival of Xylella fastidiosa in vitro and in potted grapevines. Plant Dis. 85:1230-1234. [DOI] [PubMed] [Google Scholar]
- 14.Feil, H., W. Feil, and S. E. Lindow. 2007. Contribution of fimbrial and afimbrial adhesins of Xylella fastidiosa to attachment to surfaces and virulence to grape. Phytopathology 97:318-324. [DOI] [PubMed] [Google Scholar]
- 15.Guilhabert, M. R., and B. C. Kirkpatrick. 2005. Identification of Xylella fastidiosa antivirulence genes: hemagglutinin adhesins contribute to X. fastidiosa biofilm maturation and colonization and attenuate virulence. Mol. Plant-Microbe Interact. 18:856-868. [DOI] [PubMed] [Google Scholar]
- 16.Guilhabert, M. R., L. M. Hoffman, D. A. Mills, and B. C. Kirkpatrick. 2001. Transposon mutagenesis of Xylella fastidiosa by electroporation of Tn5 synaptic complexes. Mol. Plant-Microbe Interact. 14:701-706. [DOI] [PubMed] [Google Scholar]
- 17.Hasman, H., M. A. Schembri, and P. Klemm. 2000. Antigen 43 and type 1 fimbriae determine colony morphology of Escherichia coli K-12. J. Bacteriol. 182:1089-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hélaine, S., E. Carbonnelle, L. Prouvensier, J. L. Beretti, X. Nassif, and V. Pelicic. 2005. PilX, a pilus-associated protein essential for bacterial aggregation, is a key to pilus facilitated attachment of Neisseria meningitidis to human cells. Mol. Microbiol. 55:65-77. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins, D. L., and A. H. Purcell. 2002. Xylella fasidiosa: cause of Pierce's disease of grapevine and other emergent diseases. Plant Dis. 86:1056-1066. [DOI] [PubMed] [Google Scholar]
- 20.Hopkins, D. L. 1981. Seasonal concentration of the Pierce's disease bacterium in grapevine stems, petioles, and leaf veins. Phytopathology 71:415-418. [Google Scholar]
- 21.Huber, B., K. Riedel, M. Hentzer, A. Heydorn, A. Gotschlich, M. Givskov, S. Molin, and L. Eberl. 2001. The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology 147:2517-2528. [DOI] [PubMed] [Google Scholar]
- 22.Kirn, T. J., M. J. Lafferty, C. M. P. Sandoe, and R. K. Taylor. 2000. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae. Mol. Microbiol. 35:896-910. [DOI] [PubMed] [Google Scholar]
- 23.Knutton, S., R. K. Shaw, R. P. Anantha, M. S. Donnenberg, and A. A. Zorgani. 1999. The type IV bundle-forming pilus of enteropathogenic Escherichia coli undergoes dramatic alterations in structure associated with bacterial adherence, aggregation and dispersal. Mol. Microbiol. 33:499-509. [DOI] [PubMed] [Google Scholar]
- 24.Lambais, M. R., M. H. Goldman, L. E. Camargo, and G. H. Goldman. 2000. A genomic approach to the understanding of Xylella fastidiosa pathogenicity. Curr. Opin. Microbiol. 3:459-462. [DOI] [PubMed] [Google Scholar]
- 25.Li, Y., G. Hao, C. D. Galvani, Y. Meng, L. De La Fuente, H. C. Hoch, and T. J. Burr. 2007. Type I and type IV pili of Xylella fastidiosa affect twitching motility, biofilm formation, and cell-cell aggregation. Microbiology 153:719-726. [DOI] [PubMed] [Google Scholar]
- 26.Meng, Y., Y. Li, C. D. Galvani, G. Hao, J. N. Turner, T. J. Burr, and H. C. Hoch. 2005. Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. J. Bacteriol. 187:5560-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman, K. L., R. P. P. Almeida, A. H. Purcell, and S. E. Lindow. 2003. Use of a green fluorescent strain for analysis of Xylella fastidiosa colonization of Vitis vinifera. Appl. Environ. Microbiol. 69:7319-7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy, J. D., S. L. Reddy, D. L. Hopkins, and D. W. Gabriel. 2007. TolC is required for pathogenicity of Xylella fastidiosa in Vitis vinifera grapevines. Mol. Plant-Microbe Interact. 20:403-410. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg, M., D. Gutnick, and E. Rosenberg. 1980. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 9:29-33. [Google Scholar]
- 30.Schar-Zammaretti, P., and J. Ubbnik. 2003. The cell wall of lactic acid bacteria: surface constituents and macromolecular conformations. Biophys. J. 85:4076-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schembri, M. A., G. Christiansen, and P. Klemm. 2001. FimH-mediated autoaggregation of Escherichia coli. Mol. Microbiol. 41:1419-1430. [DOI] [PubMed] [Google Scholar]
- 32.Schembri, M. A., M. Givskov, and P. Klemm. 2002. An attractive surface: gram-negative bacterial biofilms. Sci. STKE. http://www.stke.org/cgi/content/full/OC_sigtrans;2002/132/re6. [DOI] [PubMed]
- 33.Shapiro, J. A. 1998. Thinking about bacterial populations as multicellular organisms. Annu. Rev. Microbiol. 52:81-104. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland, I. W. 2001. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3-9. [DOI] [PubMed] [Google Scholar]
- 35.Swanson, J., S. J. Kraus, and E. C. Gotschlich. 1971. Studies on gonococcus infection: i) pili and zones of adhesion: their relation to gonococcal growth patterns. J. Exp. Med. 134:886-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyson, G. E., B. J. Stojanovic, R. F. Kuklinski, T. J. Divittorio, and M. L. Sullivan. 1985. Scanning electron microscopy of Pierce's disease bacterium in petiolar xylem of grape leaves. Phytopathology 75:264-269. [Google Scholar]
- 37.Van Sluys, M. A., M. C. de Oliveira, C. B. Monteiro-Vitorello, C. Y. Miyaki, L. R. Furlan, L. E. Camargo, A. C. da Silva, D. H. Moon, M. A. Takita, E. G. Lemos, M. A. Machado, M. I. Ferro, F. R. da Silva, M. H. Goldman, G. H. Goldman, M. V. Lemos, H. El-Dorry, S. M. Tsai, H. Carrer, D. M. Carraro, R. C. de Oliveira, L. R. Nunes, W. J. Siqueira, L. L. Coutinho, E. T. Kimura, E. S. Ferro, R. Harakava, E. E. Kuramae, C. L. Marino, E. Giglioti, I. L. Abreu, L. M. Alves, A. M. do Amaral, G. S. Baia, S. R. Blanco, M. S. Brito, F. S. Cannavan, A. V. Celestino, A. F. da Cunha, R. C. Fenille, J. A. Ferro, E. F. Formighieri, L. T. Kishi, S. G. Leoni, A. R. Oliveira, V. E. Rosa, Jr., F. T. Sassaki, J. A. Sena, A. A. de Souza, D. Truffi, F. Tsukumo, G. M. Yanai, L. G. Zaros, E. L. Civerolo, A. J. Simpson, N. F. Almeida, Jr., J. C. Setubal, and J. P. Kitajima. 2003. Comparative analyses of the complete genome sequences of Pierce's disease and citrus variegated chlorosis strains of Xylella fastidiosa. J. Bacteriol. 185:1018-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vuopio-Varkila, J., and G. K. Schoolnik. 1991. Localized adherence by enteropathogenic Escherichia coli is an inducible phenotype associated with the expression of new outer membrane proteins. J. Exp. Med. 174:1167-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wells, J. M., B. C. Raju, H.-Y. Hung, W. G. Weisburg, L. Mandelco-Paul, and D. J. Brenner. 1987. Xylella fastidiosa gen. nov., sp. nov.: gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int. J. Syst. Bacteriol. 37:136-143. [Google Scholar]
- 40.Zhang, P., B. B. Chomel, M. K. Schau, J. S. Goo, S. Droz, K. L. Kelminson, S. S. George, N. W. Lerche, and J. E. Koehler. 2004. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc. Natl. Acad. Sci. USA 101:13630-13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.