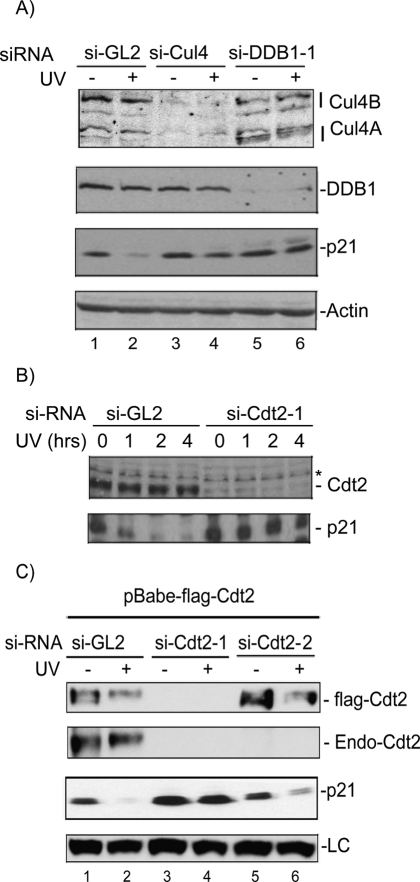
Figure 2.
The CRL4Cdt2 E3 ubiquitin ligase is required to efficiently degrade p21 in UV-irradiated cells. (A) si-RNA knockdown of Cul4A/B or DDB1 prevents p21 degradation after UV. Western blot from nonirradiated or UV-irradiated (20 J/m2) HCT116p53−/− after knockdown of Cul4A and B (si-Cul4), DDB1 (si-DDB1-1), or control siRNA (si-GL2). Actin is shown as a loading control. (B) siRNA knockdown of the DCAF Cdt2 prevents p21 degradation by UV. Western blot of HCT116p53−/− after knockdown of Cdt2 or control si-GL2, UV-irradiated (20 J/m2) and harvested after the indicated hours post-irradiation. (*) A cross-reacting band in the anti-Cdt2 blot that serves as a loading control. (C) Ectopic expression of si-RNA-resistant Cdt2 restores the UV-induced degradation of p21. U2OS cells stably expressing Flag-epitope-tagged Cdt2 (without the 3′UTR) are transfected with either si-GL2 or siRNA targeting the ORF (si-Cdt2-1), or the 3′UTR of Cdt2 (si-Cdt2-2) and UV-irradiated 6 h prior to harvesting. The anti-Cdt2 blot shows endogenous Cdt2 (Endo-Cdt2). An anti-Flag blot shows the exogenously expressed Cdt2 and its efficient silencing by si-Cdt2-1 but not si-Cdt2-2. Exogenous Cdt2 was not shown in the same blot because it overlaps with a cross-reactive band that runs higher than the endogenous protein. (LC) A cross-reacting band in the anti-p21 blot serving as a loading control.