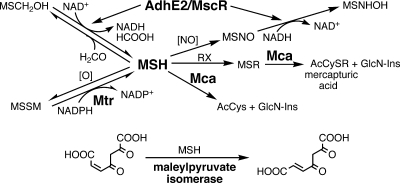
FIG. 8.
Summary of well-characterized MSH-dependent reactions where MSH is either a substrate or cofactor. MSH is the redox buffer for Actinobacteria, is oxidized by cellular oxidants, [O], and is maintained in a highly reduced status by MSH disulfide reductase (Mtr). Formaldehyde (HCOOH) and MSNO are converted to formate and MSH sulfinamide (MSONH2), respectively, by the alcohol dehydrogenase (AdhE2)/MSNO reductase (MscR). Thiol-reactive drugs form MSR that are cleaved by MSH S-conjugate amidase (Mca) to produce the N-acetyl-CyS-conjugate (AcCySR), which is excreted. The pseudodisaccharide GlcN-Ins is retained in the cell and reenters the MSH biosynthesis pathway. Another important function of Mca is the degradation of MSH to provide cysteine (from acetylcysteine [AcCys]) and GlcN-Ins as needed for other metabolic pathways. MSH is also required for the metabolism of 3-hydroxybenzoate; the MSH-dependent step is the isomerization of maleyl-pyruvate to fumaryl-pyruvate by maleylpyruvate isomerase (see Fig. 16 for more details).