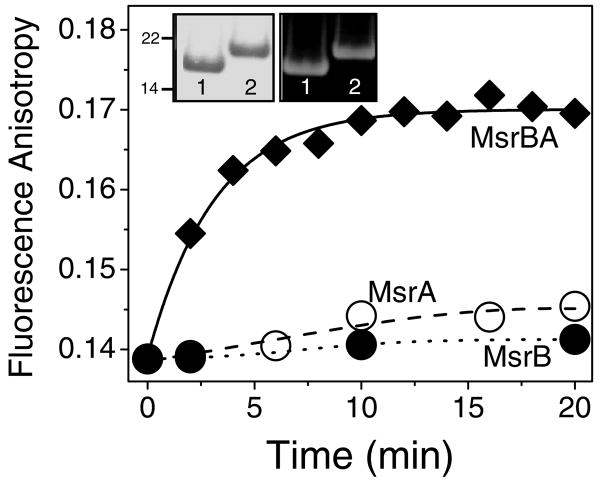
Figure 4. Restoration of CaMox Protein Fold Upon Repair of Met(SO) by MsrBA.
Steady-state anisotropies for FlAsH-labeled CaMox (1.0 μM) in the presence of MsrBA (◆), MsrA (○) and MsrB (●). Reaction mixture consists of NADPH (400 μM), thioredoxin (50 μM), thioredoxin reductase (2 μM), and indicated isoform of Msr (1.0 μM) in 50 mM HEPES (pH 7.5), 140 mM KCl, and 0.2 mM CaCl2 in a total volume of 2 mL. Initial rates associated with MsrBA-dependent increases in anisotropy are (5.9 ± 1.1) × 10−3/min, (0.53 ± 0.12) × 10−3/min, and (0.19 ± 0.02) × 10−3/min for MsrBA, MsrA and MsrB, respectively. Excitation was at 500 nm and emitted light was measured at 530 nm; slit widths were set at 5 nm. Inset: Electrophoretic mobility on SDS-PAGE of FlAsH-labeled CaM (10 μg) prior to (lane 1) and following oxidation of all nine methionines (lane 2) using a 14% Tris-Glycine gel visualized by fluorescence detection (right) or following Coomassie blue staining (left).