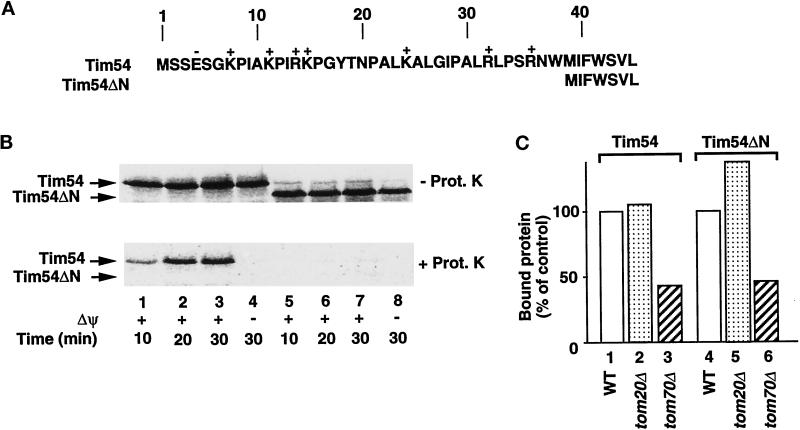
Figure 5.
Different portions of Tim54 are needed for receptor interaction and membrane translocation. (A) Generation of a construct lacking the first 38 amino acids of Tim54 (Tim54ΔN) removes a number of basic residues. Positively and negatively charged residues are indicated. Tim54ΔN initiates from the internal methionine shown at position 39. (B) Tim54ΔN is not imported into mitochondria. 35S-labeled preproteins corresponding to Tim54 (lanes 1–4) and Tim54ΔN (lanes 5–8) were incubated with mitochondria for the indicated times in the presence (lanes 1–3, 5–7) or absence (lanes 4 and 8) of a membrane potential (Δψ). After import, samples were halved and treated with (bottom panel) or without (top panel) proteinase K (Prot. K). (C) Binding of Tim54ΔN to mitochondria is inhibited by a lack of Tom70. 35S-labeled Tim54 and Tim54ΔN preproteins were arrested on the outer membrane by depleting ATP from mitochondria and reticulocyte lysates. Samples were analyzed by SDS-PAGE, and radiolabeled proteins were quantified using ImageQuant software. Binding to isolated mitochondria from wild-type cells (WT; control) was compared with binding to mitochondria lacking Tom20 (tom20Δ) or Tom70 (tom70Δ).