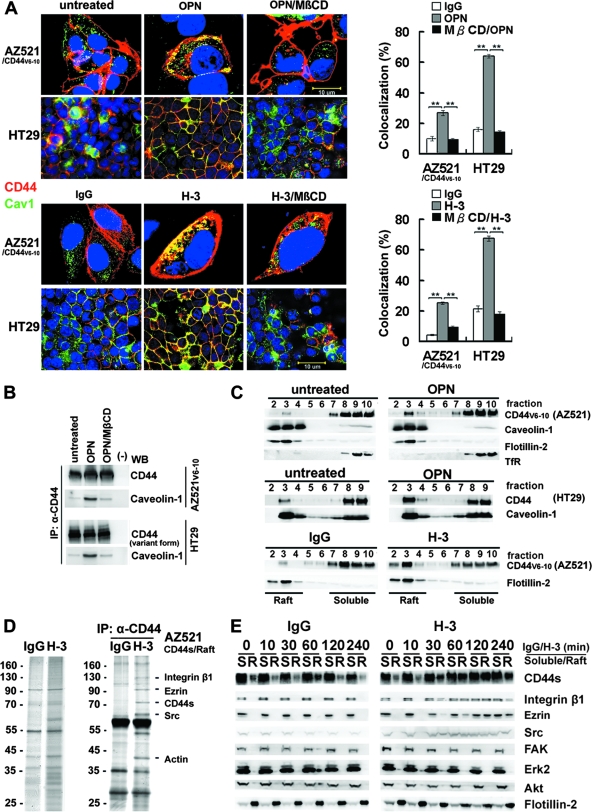
FIG. 2.
Engagement of CD44 induces relocalization of CD44, Src, and integrin β1 to lipid rafts. (A) Immunofluorescence examination of CD44 and raft marker in AZ521/CD44V6-10 and HT29 cells treated with or without OPN or H-3. For staining of CD44, cells were incubated with H-3 (20 μg/ml) for 1 h followed by fixation and labeling with Alexa 594-conjugated secondary Ab, whereas the control and OPN (10 μg/ml)-treated cells were fixed and incubated with H-3 MAb followed by labeling with Alexa 594-conjugated anti-mouse IgG. Both the stimulated and control samples were then counterstained with anti-raft marker (caveolin-1) Ab and labeled with Alexa 488-conjugated secondary Ab. Representative images taken by confocal laser microscopy are shown. In some experiments, cells were pretreated with 5 μM MβCD for 15 min prior to H-3 MAb or OPN treatment. Image J software (National Institutes of Health) was used to process the immunocytostaining signals of CD44 and raft marker (caveolin-1). The bar graph shows the percentages of colocalized signals in each set of samples expressed as means ± standard deviations. Data are representative of the images of 12 fields derived from each of three independent experiments. **, P < 0.01 (t test). (B) AZ521/CD44V6-10 (top) and HT29 (bottom) cells were pretreated with or without MβCD followed by OPN or H-3 treatment as described above. The whole-cell lysates were prepared and immunoprecipitated (IP) by anti-CD44 H-3 Ab. The immunoprecipitation complexes were subjected to Western blotting (WB) against H-3 and anti-caveolin-1 Ab, respectively. (C) AZ521/CD44V6-10 and HT29 cells were incubated in the presence and absence of OPN and H-3 MAb as described in the Fig. 1 legend. The cells were lysed in chilled 1% cold Triton X-100 buffer, and lysates were subjected to sucrose gradient fractionation. A total of 10 fractions were collected from top to bottom, and an equal volume of each fraction was subjected to Western blot analyses for proteins indicated. Fractions 2 to 4 represent the Triton X-100-insoluble raft fraction, and fractions 7 to 10 contain the Triton X-100-soluble cytoplasmic components, including transferrin receptor (TfR). (D) AZ521/CD44S cells were incubated in the presence of control IgG or H-3 MAb for 1 h. After washing, cells were lysed and Triton X-100-insoluble raft fractions were isolated. The raft fractions were pooled and immunoprecipitated by H-3 MAb. Silver stains of raft proteins (left panel) before immunoprecipitation and those immunoprecipitated by H-3 MAb (right panel) are shown after fractionation by 10% SDS-PAGE. The identities of protein signals identified by mass spectrometry are indicated. (E) Time course of CD44 engagement-induced enrichment of CD44, Src, and integrin β1 into lipid rafts was monitored in AZ521/CD44S cells. Cells were treated with H-3 MAb or control IgG and harvested at designated time points. Triton X-100-soluble (S) and -insoluble raft (R) fractions were isolated by sucrose gradient fractionation, pooled, and subjected to Western blotting for individual proteins as indicated.