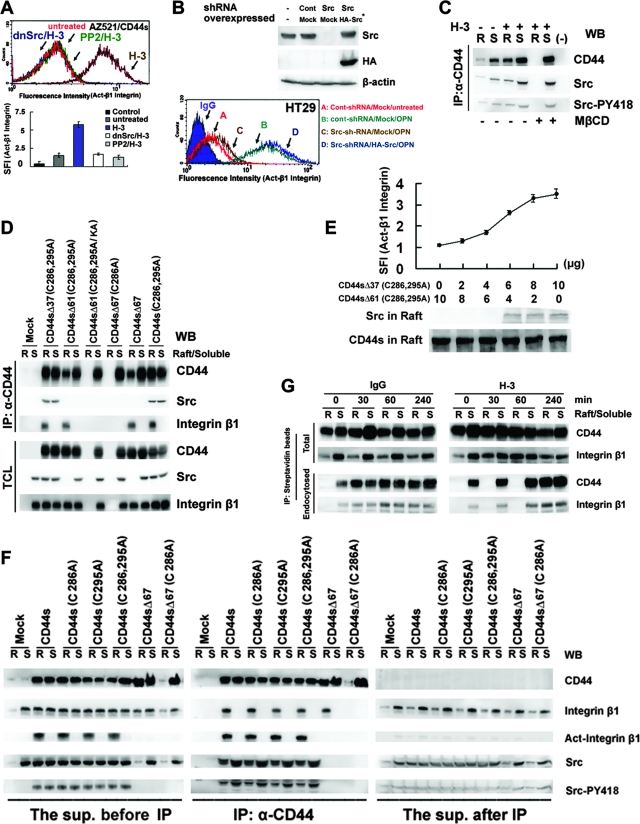
FIG. 5.
Src is cotranslocated to lipid rafts through its association with CD44 and induces integrin activation. (A) Activation of integrin β1 was monitored by flow cytometric analyses in control IgG-treated (untreated) or H-3-treated AZ521/CD44S cells that were pretreated with or without PP2 (30 μM) for 2 h or were transfected with a plasmid encoding dn-Src(K297D). CD44 engagement-mediated integrin activation was measured as described in the legend for Fig. 4B. (B) HT29 cells were infected with a lentivirus encoding a shRNA targeting Src, shRNASrc, or a control scramble shRNA, shRNACont, and cell clones stably harboring shRNASrc and shRNACont were obtained after puromycin selection. The stable cell clones were transfected with a control plasmid (Mock) or a plasmid encoding an HA epitope-tagged Src wobble mutant (Src*). Western blotting was performed to measure the expression of Src. Activation of integrin β1 was monitored after OPN treatment for 1 h. (C) Triton X-100-soluble (S) and -insoluble raft (R) fractions were isolated from H-3- and control IgG-treated AZ521/CD44S cells that were pretreated with or without MβCD. The Triton X-100-soluble and -insoluble raft fractions were pooled and immunoprecipitated (IP) by H-3 MAb or control IgG (−). Western blot (WB) analyses of CD44-associated Src and phosphorylated Src are shown. (D) The Triton X-100-soluble and -insoluble raft fractions were isolated from the individual AZ521/CD44 cell clones as described in the Fig. 1 legend and immunoprecipitated with H-3 MAb. Western blot analyses of CD44, Src, and integrin β1 in detergent-soluble (S) and -insoluble fractions (R) before immunoprecipitation and in the immunoprecipitates were performed. (E) Integrin activation and translocation of Src into lipid rafts were monitored in AZ521 cells cotransfected with plasmids expressing CD44SΔ37C286,295A and CD44SΔ61C286,295A in various combinations as indicated. Activation of integrin β1 was monitored by flow cytometric analysis as described above. Triton X-100-insoluble fractions were isolated and subjected to Western blotting for CD44 and Src. (F) AZ521/Mock and individual AZ521/CD44S cell clones were treated with H-3 MAb. Triton X-100-soluble (S) and -insoluble raft (R) fractions were isolated, pooled, and immunoprecipitated with H-3 MAb. Western blot analyses of CD44, Src, Src-pY418, integrin β1, and activated integrin β1 in the raft (R) and Triton X-100-soluble (S) fractions before immunoprecipitation (left panel), in the immunoprecipitates (middle panel), and in the supernatant (right panel) recovered after immunoprecipitation were performed, and enhanced chemiluminescence signal intensity for each probe was recorded simultaneously for the three panels. (G) AZ521/CD44S cells were labeled with biotin at 4°C, followed by further incubation with control IgG or H-3 MAb at 37°C. At time zero and 30, 60, and 240 min, cells were harvested and Triton X-100-soluble (S) and -insoluble raft (R) fractions were isolated. Biotinylated proteins in the Triton X-100-soluble and -insoluble raft fractions were precipitated using streptavidin beads, and the amounts of CD44 and integrin β1 bound to the beads were determined by Western blot analysis. To examine the internalized receptors, cells were removed to 4°C and incubated in 0.1 M glycine for an additional 30 min prior to the isolation of Triton X-100-soluble and -insoluble raft fractions followed by Western blot analysis of precipitates pulled down by streptavidin beads as described in Materials and Methods.