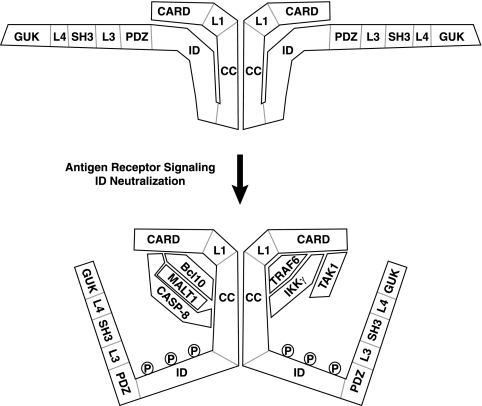
FIG. 15.
Model of the signal-induced conversion of CARD11 to an active signaling scaffold. Prior to TCR engagement, the ID of CARD11 interacts with the CARD and the coiled-coil (CC) domain to prevent the association of signaling cofactors. Although it is depicted here as an intramolecular interaction in the inhibited state, it is also possible that the ID interacts with the CARD and the coiled-coil domain of another CARD11 molecule in the oligomer. TCR signaling leads to the neutralization of ID activity, in part through the phosphorylation of the ID by PKCθ. Once the ID is neutralized, signaling cofactors can be recruited to the N-terminal portion of CARD11. The model indicates which domains of CARD11 are required for the association with the cofactors analyzed. TAK1 requires only the CARD, while Bcl10 requires the CARD and coiled-coil domain. TRAF6 requires the CARD and the L1 and coiled-coil domains, while caspase-8 and IKKγ each require the CARD and the coiled-coil domain. MALT1 is presumably recruited through interactions with Bcl10. Caspase-8 (CASP-8) requires both Bcl10 and MALT1 to associate with CARD11 during signaling. The association of TAK1, TRAF6, and IKKγ with activated CARD11 can occur in a Bcl10-independent and MALT1-independent manner. The ID does not appear to influence whether CARD11 oligomerizes, suggesting that oligomerization occurs both before and after signaling through a region of the coiled-coil domain that is not targeted by the ID. The depicted interactions between CARD11 and signaling cofactors may be direct or may require other proteins to bridge the associations.