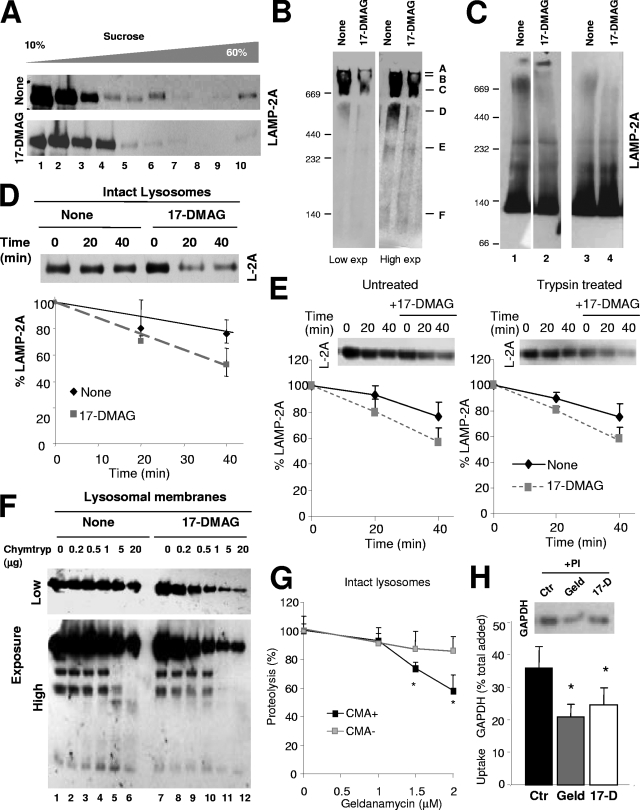
FIG. 9.
Novel role for hsp90 as a stabilizer of LAMP-2A at the lysosomal membrane. (A) Intact rat liver lysosomes with high CMA activity were incubated alone or with the geldanamycin derivative 17-DMAG. At the end of the incubation, lysosomes were subjected to sucrose density gradient centrifugation (A), native gel electrophoresis (B), or BNE (C) and immunoblotted for LAMP-2A. Two exposures (Exp) of the same immunoblot are shown in panel B to better appreciate the region corresponding to the monomeric form of LAMP-2A. Two independent experiments are shown in C (lanes on the left were run in the same gel but were cut as they were separated by irrelevant samples). (D) Resealed membranes from lysosomes preincubated or not with 17-DMAG were incubated at 37°C in an isotonic buffer, and the remaining levels of LAMP-2A at the indicated times were detected by immunoblotting. Values are expressed as percentages of the LAMP-2A at time zero and are the means ± SE of the densitometric quantification of the immunoblots from three different experiments. A representative immunoblot is shown. (E) Intact lysosomes untreated or previously preincubated with 25 μg of trypsin and with 17-DMAG as indicated were incubated (after neutralization of the protease with a trypsin inhibitor) at 37°C in an isotonic buffer, and the remaining levels of LAMP-2A at the indicated times were detected by immunoblotting. Values are expressed as described for panel D and are the means ± SE of the densitometric quantification of the immunoblots from two different experiments. (F) Proteolytic cleavage pattern of LAMP-2A in lysosomal membranes isolated from lysosomes incubated as described for panel B and treated with increasing concentrations of chymotrypsin (Chymtryp). Images of a low (top) and a high (bottom) exposure time of the immunoblot for LAMP-2A are shown. (G to H) Effect of geldanamycin (Geld) and 17-DMAG (17-D) on CMA of GAPDH by isolated lysosomes. Panel G shows the effect of increasing concentrations of geldanamycin on the degradation of GAPDH by intact lysosomes with high (+) or low (−) CMA activity. Values are expressed as percentages of the degradation in untreated lysosomes to be able to compare the samples despite the low rates of degradation observed in the CMA− lysosomes and are the means + SE of the results from two different experiments with triplicate samples. In panel H, untreated lysosomes (control; Ctr) or lysosomes treated with the indicated hsp90 inhibitors and preincubated or not with protease inhibitors (PI) were incubated with GAPDH. Lysosomes were collected by centrifugation, and the amount of GAPDH associated with each sample was determined by SDS-PAGE and immunoblotting. Uptake was calculated after densitometric quantification as the difference between association and binding. Values are expressed as percentages of total GAPDH added and are the means + SE of six different experiments. *, P < 0.01.