FIG. 8.
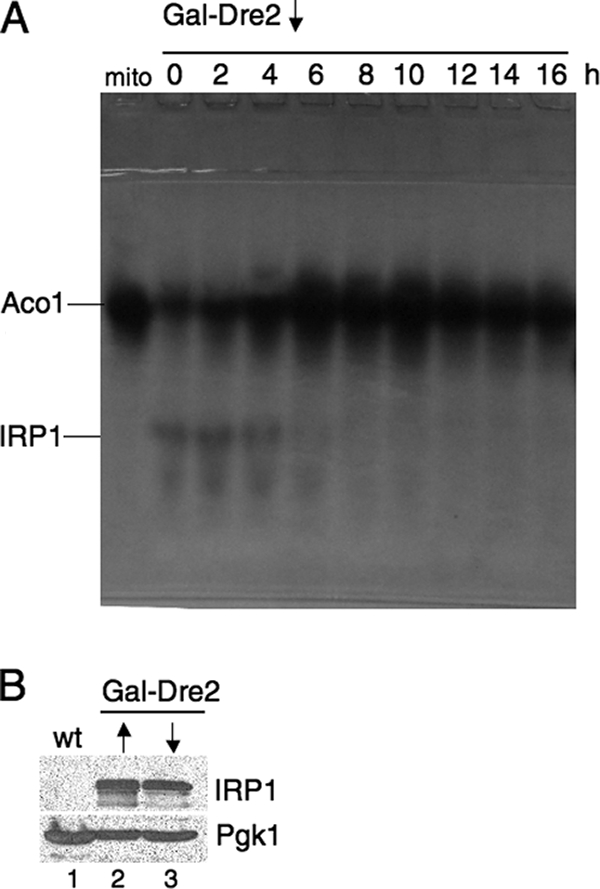
Dre2-depleted cells lack IRP1 activity. The Gal-Dre2 strain (98-67) was transformed with YCplac22-GPDprom-IRP1 (plasmid 18-3). Cells were grown in the presence of galactose (defined medium with 2% raffinose and 0.5% galactose), centrifuged, and resuspended in the absence of galactose for the indicated times, thereby depleting Dre2. (A) Depletion time course: cells were collected immediately following shift (zero time) and 2, 4, 6, 8, 10, 12, 14, and 16 h later. Lysates generated by zymolyase digestion were resolved by native gel electrophoresis, and an in-gel assay for aconitase was performed. Mitochondrial aconitase activity (Aco1) and plasmid-expressed cytoplasmic aconitase activity (IRP1) are indicated. The leftmost lane (“mito”) contains 100 μg of purified mitochondria from untransformed parental strain YPH499. (B) Immunoblots for IRP1 protein. Whole-cell lysates from untransformed parental strain YPH499 (lane 1), induced Gal-Dre2 expressing IRP1 (lane 2), and noninduced Gal-Dre2 expressing IRP1 following 16 h of growth (lane 3) were analyzed by SDS-PAGE and immunoblotted with anti-IRP1 or cytoplasmic marker Pgk1 as a control (100 μg of protein for each lane). wt, wild type.
