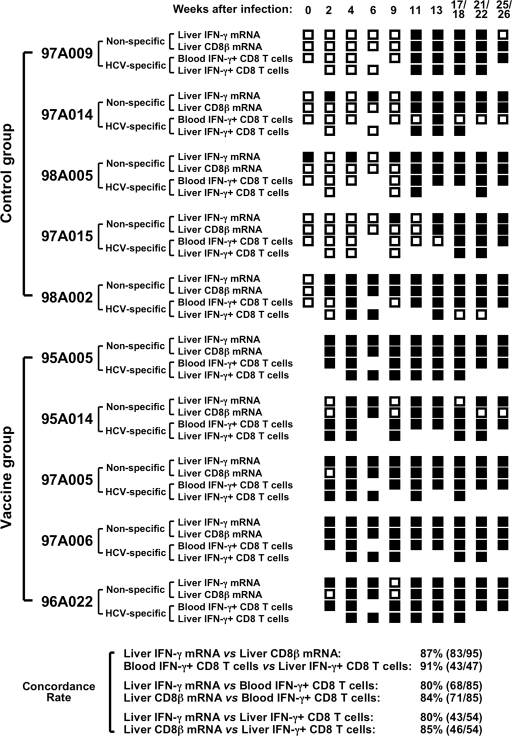
FIG. 3.
Use of molecular (CD8β and IFN-γ mRNA levels) and functional (IFN-γ production) assays to assess the onset of HCV-specific T-cell responses in blood and liver. Intrahepatic IFN-γ and CD8β mRNA levels during acute HCV infection (defined as “non-specific” in the figure) were considered positive if they were more than twofold over preinfection levels. The frequency of HCV-specific, IFN-γ-producing CD8 T cells in blood and liver (defined as “HCV-specific” in the figure) was determined by intracellular cytokine staining using peptides overlapping the HCV nonstructural proteins as previously reported (6). HCV-specific IFN-γ responses of ex vivo-analyzed peripheral blood CD8 T cells were considered positive if greater than 100 HCV-specific IFN-γ+ CD3+ CD8+ cells/106 lymphocytes were detected (6). IFN-γ responses of in vitro-expanded intrahepatic CD8 T cells were considered positive if the response in the presence of overlapping HCV peptides was more than three times higher than the response in the absence of peptides (6). Positive responses are indicated by filled squares and negative responses are indicated by open squares for each individual chimpanzee and time point throughout the study. Concordance rates indicate the number of concordant immune response parameters/(number of total comparisons between two immune response parameters) × 100, where concordance means that two immune response parameters are either both positive or both negative at the same time point.