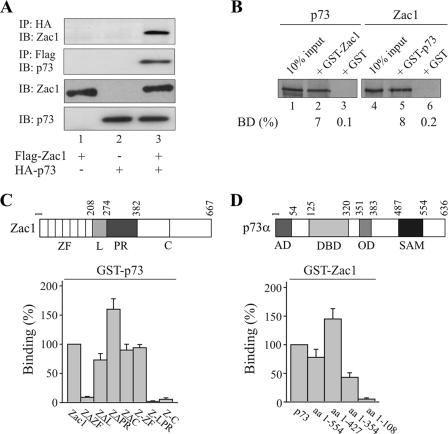
FIG. 2.
Zac1 and p73 interact through their DNA-binding domains. (A) Coimmunoprecipitation experiments. Flag-Zac1 (0.5 μg) and hemagglutinin (HA)-p73 (1 μg) were transfected alone or in combination into Saos-2 cells, and WCE (1 mg) were immunoprecipitated with anti-HA or -Flag antibodies. Immunoprecipitates were immunoblotted and tested with the indicated antibodies. +, presence; −, absence. (B to D) GST pull-down assays. Adjusted amounts of GST-Zac1, GST-p73, or GST alone were incubated with equal amounts of in vitro-translated Zac1 or p73 (B). The fraction of the input (100%) bound by each GST protein [BD (%)] is indicated. (C) Equal amounts of in vitro-translated Zac1 proteins, containing deletions of the zinc finger (ZΔZF), linker (ZΔL), and proline-rich (ZΔPR) or the C-terminal (ZΔC) domains, were each incubated with GST-p73 or GST alone. Similarly, equal amounts of the isolated zinc finger (Z-ZF), linker proline-rich (Z-LPR), or C-terminal (Z-C) domains were each incubated with GST-p73 or GST alone. Binding (%) by Zac1 was set to 100%. (D) Equal amounts of in vitro-translated p73 proteins containing successive deletions of the carboxyl terminus were incubated with GST-Zac1 or GST alone. Binding (%) by p73 was set to 100%. AD, activation domain; DBD, DNA-binding domain; OD, oligomerization domain; SAM, sterile alpha motif.