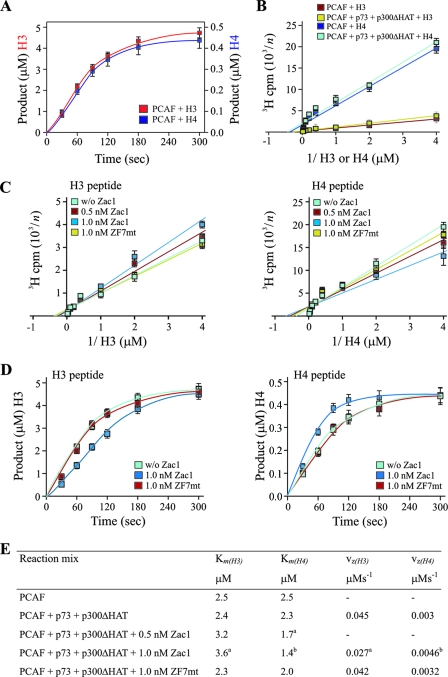
FIG. 7.
Zac1 regulates PCAF's substrate affinities and catalytic activity. (A to D) PCAF in vitro HAT assays. (A) Kinetics of acetylation reactions for saturating concentrations of histone H3 or H4 peptides. The linear range of HAT enzymatic reactions was determined between 30 and 80 s. (B) Saturation analysis for various concentrations of H3 or H4 peptides in the absence or presence of p73 and p300ΔHAT, which do not alter PCAF's substrate affinities. (C) Affinities for H3 or H4 peptides in the presence of p73 and p300ΔHAT and in the absence (w/o) or presence of the indicated concentrations of Zac1 or ZF7-mutated Zac1 (ZF7mt). Zac1 significantly increases and decreases the affinity for histone H4 and H3, respectively. In contrast, ZF7-mutated Zac1 does not alter PCAF's substrate affinities. The y axis data in panels B and C are presented reciprocally, with the numbers on the y axis corresponding to n (e.g., a y axis value of 2 represents 500 cpm). (D) HAT reaction progress curves for H3 and H4 peptides in the presence of p73 and p300ΔHAT and in the absence (w/o) or presence of the indicated concentrations of Zac1 or ZF7-mutated Zac1 (ZF7mt). Zac1 significantly increases and decreases histone H4 and H3 catalytic activities, respectively. In contrast, ZF7-mutated Zac1 does not alter PCAF's catalytic activity. (E) Overview of deduced substrate affinities (Km) and maximal vz (Student's t test; a, P < 0.05; b, P < 0.01).