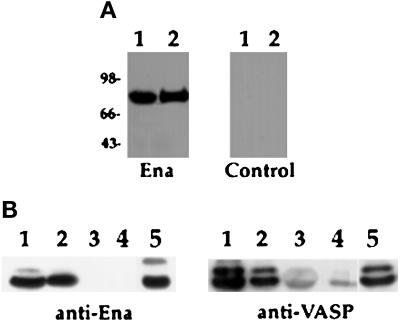
Figure 4.
Binding of Ena and VASP proteins to zyxin and mapping of the binding domain in zyxin. (A) Ena and VASP overlays were carried out essentially as previously described (Reinhard et al., 1995a). Fifty micrograms of total protein from human platelets (lane 1) and 100 ng of purified porcine platelet zyxin (lane 2) were separated by SDS-PAGE and transferred to nitrocellulose. The blot was overlaid with Ena purified from baculovirus-infected cells, and bound Ena was detected by the anti-Ena antibody followed by 125I-protein A (left panel). The control (right panel) was treated identically, except that the overlay solution contained no Ena protein. Ena protein bound to an 83-kDa protein present in platelet lysates (lane 1), which comigrates with full-length zyxin (lane 2), whereas the control panel gave no detectable signal (right panel). (B) S2 cells were transfected with Ena or VASP, and proteins were bound to GST fusion proteins as follows: full-length chicken zyxin (lane 1), zyxin proline domain (lane 2), zyxin LIM domains (lane 3) (Schmeichel and Beckerle, 1994), and GST alone (lane 4). Retained proteins were detected with anti-Ena N-terminal (left panel) or anti-VASP (right panel) polyclonal antibodies. Both proteins bound to full-length zyxin and to the zyxin proline domain (lanes 1 and 2). Neither protein bound to the zyxin LIM domain (lane 3). The faint signal seen in lanes 3 and 4 in the anti-VASP blot is due to background binding between VASP and the GST fusion proteins. Aliquots of transfected cell lysates were analyzed for expression of Ena or VASP protein (lanes 5). These results are representative of three independent experiments.