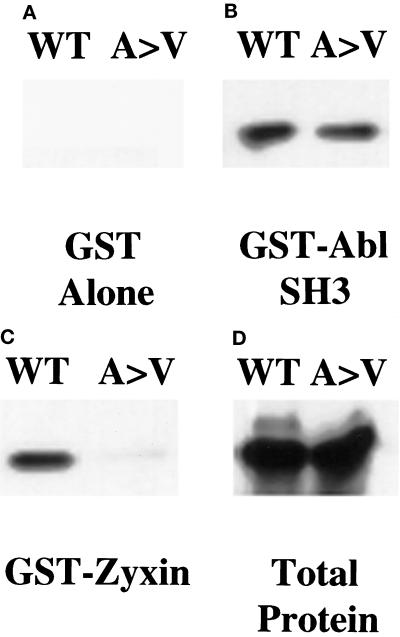
Figure 5.
ena 210 mutation disrupts zyxin binding. Full-length wild-type Ena (WT) or ena210 (A97V) proteins were prepared using an in vitro transcription–translation reaction and bound to GST alone (A), GST-AblSH3 (B), or GST-zyxin (C). Retained proteins were detected with anti-Ena N-terminal polyclonal antibody. Both proteins were expressed equally well (D). As expected, the GST negative control did not bind detectably to either protein (A). Both proteins bound equally well to the GST-AblSH3 domain (B). The wild-type Ena protein bound to the GST-zyxin protein, whereas binding to the A97V mutant protein was reduced. (C) These results are representative of three independent experiments.