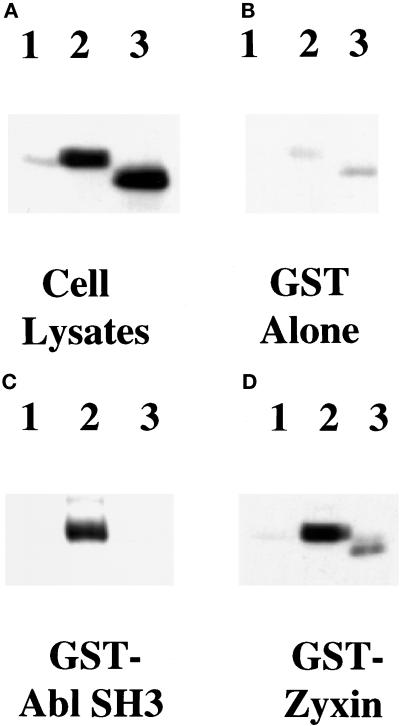
Figure 8.
Ena K636Stop is impaired in its ability to bind zyxin and the Abl-SH3 domain. S2 cells were transfected with no DNA (lanes 1), full-length Ena (lanes 2), or Ena K636Stop (lanes 3) expression vectors. Transfected cell lysates were incubated with GST-Zyxin (D) or Abl SH3 (C) domain fusion proteins or with GST alone as a negative control (B). Cells were lysed, and aliquots of the cell lysates were compared to confirm that equal amounts of the full-length and truncated Ena proteins were expressed (A). Lysates were incubated with the GST fusion proteins, and retained proteins were detected with anti-Ena N-terminal polyclonal antibody. Little protein bound to the GST negative control (B). A decrease in binding of the truncated Ena K636Stop protein was seen with both zyxin and Abl SH3 when compared with the full-length Ena protein (C and D, compare lanes 2 and 3). These results are representative of three independent experiments.