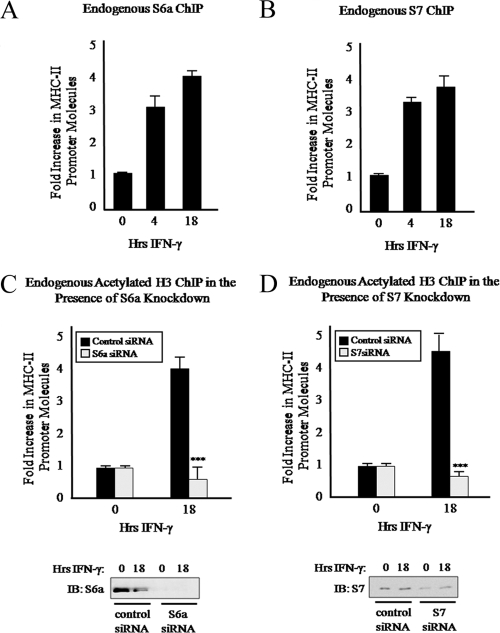
FIG. 9.
Additional 19S ATPases also mediate MHC-II promoter histone acetylation. (A and B) 19S ATPases S6a and S7 are recruited to the MHC-II proximal promoter upon IFN-γ stimulation. ChIP assays were carried out with HeLa cells stimulated with IFN-γ for 0 to 18 h. Lysates were subjected to IP with control antibody or antibody to endogenous S6a (A) or S7 (B), and associated DNA was isolated and analyzed via real-time PCR as described for Fig. 2B. IP values are presented as increases in the MHC-II promoter DNA relative to unstimulated S6a or S7 IP sample values. Control IP values were (1.2 ± 0.2)-fold. Control and ATPase IP values represent the mean ± SEM of two to four independent experiments. (C and D) S6a and S7 knockdowns diminish H3 acetylation at the MHC-II proximal promoter. ChIP assays were carried out with HeLa cells transfected with S6a, S7, or control siRNA duplexes and 24 h later stimulated with IFN-γ for 0 to18 h. Ten percent of the total cell volume was lysed and analyzed by Western blotting for S6a or S7 knockdown (bottom). The remaining fractions of cells were subjected to a ChIP assay. Lysates were subjected to IP with control antibody or antibody to endogenous acetylated H3, and associated DNA was isolated and analyzed via real-time PCR as described for Fig. 2B. IP values are presented as increases in the MHC-II promoter DNA relative to unstimulated acetylated histone H3 IP sample values. Control IP values were (0.85 ± 0.4)-fold. Control and acetylated histone H3 IP values represent the mean ± SEM of two independent experiments. ***, P < 0.001 versus control siRNA.