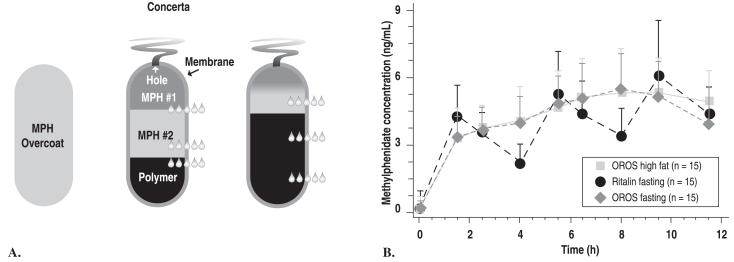
Figure 3.
Proof-of-product study: PK profiles for OROS-MPH (Concerta) and IR-MPH (Ritalin)
(left side) A. Greenhill LL, et al. J Am Acad Child Adolesc Psychiatry 2003;42:1234
(right side) B. Swanson JM, et al. Arch Gen Psychiatry 2003;60:204. Copyright © 2003, American Medical Association. All rights reserved.
3B. The pharmacokinetic profiles from a 3-way crossover study of immediate release OROS-methylphenidate hydrochloride administered with a (high-fat breakfast) and without (fasting) food, and tid (3 times daily)-methylphenidate administered in the fasting state. OROS is a new oral once-a-day formulation to deliver methylphenidate by osmotic pump process based on OROS technology (ALZA Corp, Mountain View, Calif).