Figure 6.
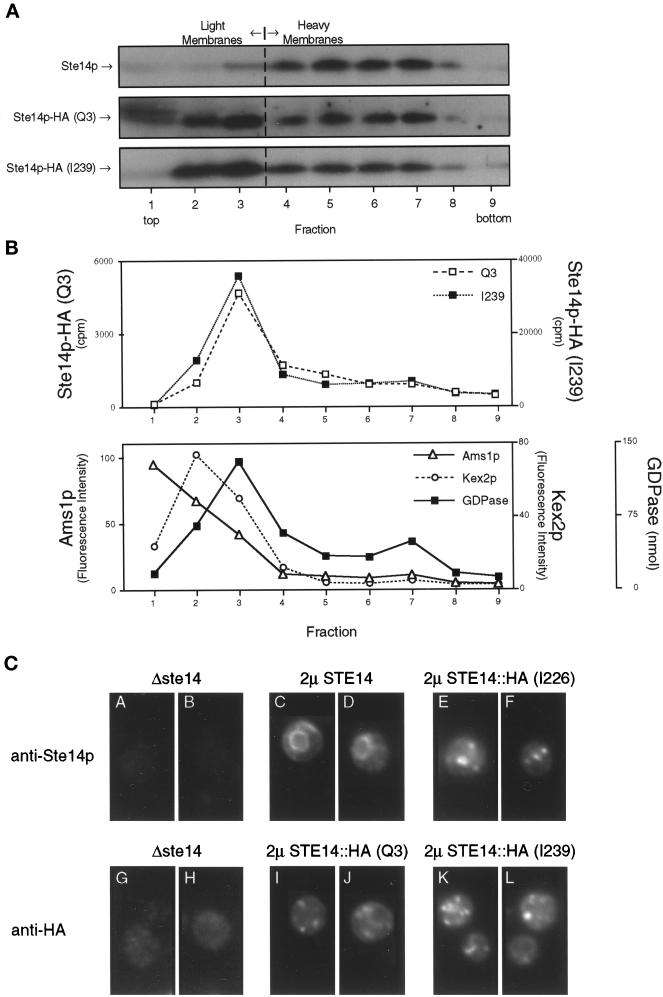
The HA epitope tag mislocalizes Ste14p, as shown by subcellular fractionation and immunofluorescence. (A) Total yeast lysates derived from strains expressing CEN levels of wild-type and epitope-tagged (Q3 and I239) Ste14p (SM3185, SM3187, and SM3189, respectively) were subjected to fractionation on a sucrose step gradient similar to that described for Figure 4. Equivalent volumes from all gradient fractions were subjected to SDS-PAGE, transferred to nitrocellulose, and probed for Ste14p and epitope-tagged Ste14p. Fractions from wild-type and Q3 were probed with anti-Ste14p antiserum, whereas I239 fractions were probed with the anti-HA antibody. As expected, wild-type Ste14p fractionates with the heavy membranes (fractions 5–7). For fractions containing either N- or C-terminal epitope-tagged Ste14p, the majority of the methyltransferase cofractionates with light membranes (fractions 2–3). (B) Equivalent volumes from the Q3- and I239-derived fractions were assayed for methyltransferase activity and three light membrane activities. The Q3 and I239 methyltransferase activities cofractionate with Golgi GDPase activity and not with vacuolar α-d-mannosidase (Ams1p) or trans-Golgi network Kex2p activities. The three marker activities are averaged from the Q3 and I239 gradients. (C) Indirect immunofluorescence of Ste14p and epitope-tagged Ste14p is shown. Fixed and permeabilized cells were stained with either a 1:2000 dilution of rabbit anti-Ste14p antiserum that had been depleted previously of nonspecific antibodies (panels A-F) or a 1:2000 dilution of mouse anti-HA antiserum (12CA5) (panels G-L), followed by secondary decoration with either Cy3-conjugated goat anti-rabbit antiserum (panels A-F) or Cy3-conjugated goat anti-mouse antiserum (panels G-L).