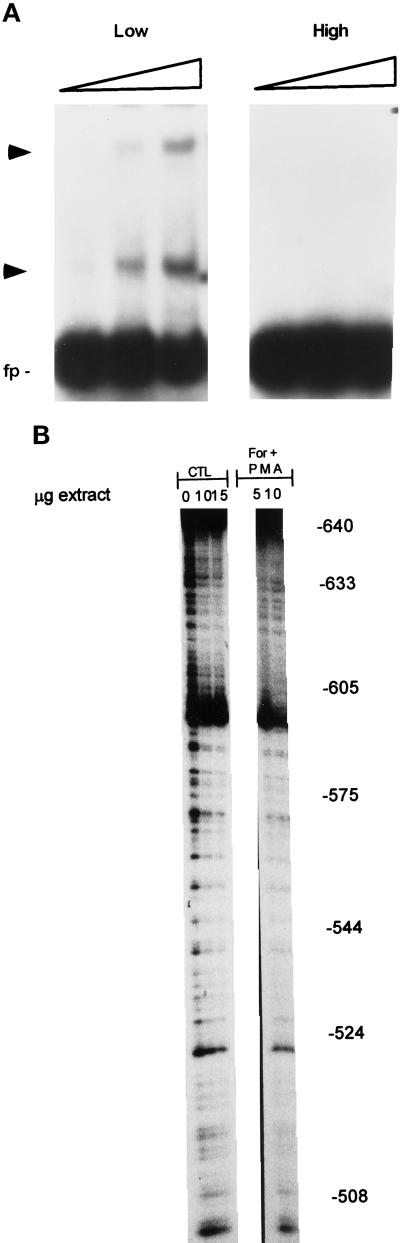
Figure 6.
Protein binding to the upstream region of FGF-2 promoter. (A) EMSA of nuclear extracts from astrocytes grown at high and low density. Nuclear extracts were prepared from astrocytes grown at high (3.5 × 104 cells/cm2, 100% confluence) or low (1.2 × 103 cells/cm2, 40–50% confluence) density in 10% FBS, and binding reactions were performed as described in MATERIALS AND METHODS. One, 2.5, or 5 μg of nuclear proteins were used for each culture condition. The target DNA contained nucleotides corresponding to FGF-2 promoter sequences −650 to −512 bp and additional sequences downstream from GFRR (−511 to −453 bp) that do not bind nuclear proteins from subconfluent or confluent astrocytes (Moffett et al., 1996). (B) DNase I footprinting of FGF-2 promoter regions (coding strand) involved in growth factor and second messenger stimulation. Variable amounts of nuclear proteins (0–15 μg) from control astrocytes or astrocytes treated with second messenger stimulators were incubated with 32P-labeled FGF-2 promoter DNA followed by limited digestion with DNase I. The reaction products were then subjected to electrophoresis in 7% sequencing gels. The DNA fragments were visualized using autoradiography. The numbers represent location of bases within the FGF-2 gene promoter. Scanning showed that intensity of bands in protected subregions −624/−600, −575/−556, and −520/−510 nt was reduced by 48, 57, and 80, respectively, when 10 μg of PMA + forskolin extract was compared with control extract. In contrast, cell stimulation with PMA and forskolin had little (−10%) reducing effect on band intensity in the −595/−580-nt subregion and an increasing effect (+60%) in the −630/−625 subregion.