Table 2.
Tri-, tetra-, and pentachlorophenols as substrates for hSULT2A1.
| Molecule | Structure | Sulfation at 10 µM (nmol/min/mg) | Sulfation at 50 µM (nmol/min/mg) | Relative activity (%)a |
|---|---|---|---|---|
| 2,3,4-Trichlorophenol | 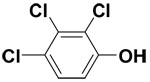 |
10.1 ± 1.0 | 30.9 ± 0.3 | 55.2 |
| 2,3,5-Trichlorophenol | 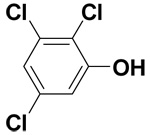 |
14.6 ± 0.8 | 47.7 ± 0.7 | 106.4 |
| 2,3,6-Trichlorophenol | 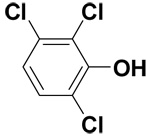 |
10.5 ± 0.7 | 28.4 ± 0.8 | 65.9 |
| 2,4,5-Trichlorophenol | 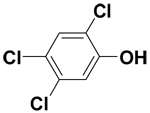 |
14.4 ± 1.3 | 46.0 ± 1.1 | 81.7 |
| 2,4,6-Trichlorophenol | 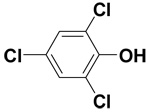 |
9.3 ± 0.1 | 26.2 ± 0.2 | 44.7 |
| 3,4,5-Trichlorophenol | 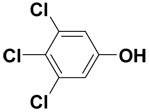 |
12.1 ± 1.0 | 38.2 ± 0.7 | 89.3 |
| 2,3,4,5-Tetrachlorophenol | 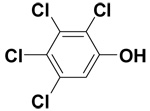 |
16.0 ± 0.5 | 67.1 ± 1.7 | 160.9 |
| 2,3,4,6-Tetrachlorophenol | 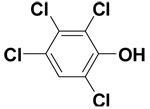 |
10.1 ± 1.5 | 21.6 ± 0.2 | 51.2 |
| 2,3,5,6-Tetrachlorophenol | 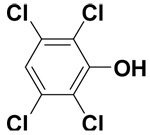 |
4.0 ± 0.3 | 12.9 ± 2.6 | 29.5 |
| Pentachlorophenol | 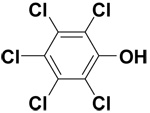 |
13.2 ± 0.2 | 48.0 ± 0.9 | 91.2 |
Relative activity is expressed as a percentage based on the ratio of the rate of sulfation of the chlorinated phenol at 50 µM to the rate of sulfation of 50 µM DHEA under the same assay conditions.
