Table 1.
Optimization Studiesa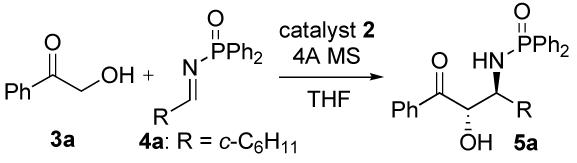
| entry | 3a (equiv) | 4a (equiv) | cat. 2 (mol%) | temp (°C) | time (h) | yieldb (%) | drc (anti:syn) | eed (%) (anti) |
|---|---|---|---|---|---|---|---|---|
| 1e | 1.4 | 1 | 2a (3.5) | 23 | 17 | 62 | 1:1 | ND |
| 2e | 1.4 | 1 | 2a (3.5) | -5 | 17 | 66 | 1:1 | (-)-67 |
| 3 | 1.4 | 1 | 2a (3.5) | -25 | 14 | 62 | 5:1 | (-)-96 |
| 4 | 2 | 1 | 2a (5.0) | -30 | 36 | 86 | 5:1 | (-)-94 |
| 5 | 2 | 1 | 2bf (5.0) | -30 | 36 | 80 | 6:1 | (+)-96 |
| 6 | 2 | 1 | 2c (5.0) | -30 | 36 | 75 | 4:1 | ND |
| 7 | 2 | 1 | 2a (3.5) | -30 | 24 | 86 | 5:1 | (-)-94 |
| 8 | 2 | 1 | 2a (2.5) | -30 | 36 | 90 | 5:1 | (-)-92 |
To mixture of catalyst 2, ketone 3a and 4Å MS in THF was added imine 4a in THF at the temperature shown in the Table.
Isolated yield.
Determined by the 1H NMR of the crude mixture.
Determined utilizing chiral HPLC.
To suspension of 3a, imine 4a, and 4Å MS in THF was added the catalyst 2 in THF.
(R,R)-catalyst 2b was used.
ND = not determined.
