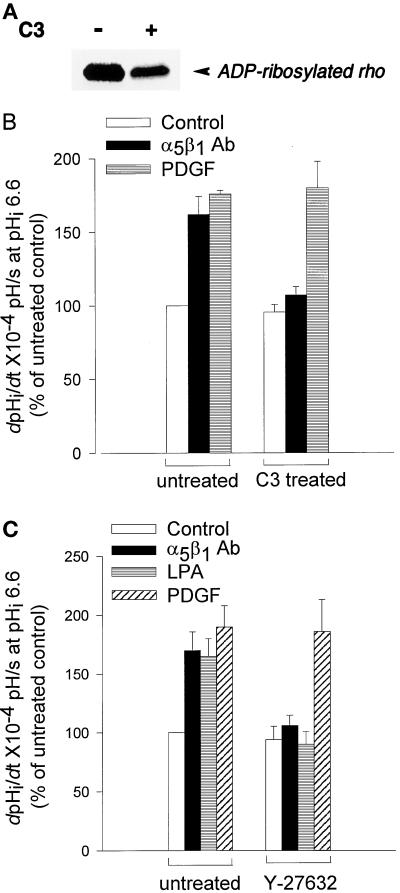
Figure 9.
RhoA and p160ROCK mediate activation of NHE1 by integrins. (A) The decrease in ADP-ribosylation substrate in C3 transferase–treated CCL39 cells. Cells were maintained in the absence or presence of C3 transferase (7.5 μg/ml) for 60 h. The remaining ADP-ribosylation substrate (unmodified RhoA) in homogenized cells was ADP-ribosylated in vitro with C3 transferase in the presence of [32P]NAD+ and was analyzed by SDS-PAGE and autoradiography. (B and C) The rate of pHi recovery (dpHi/dt) determined in a HEPES buffer in untreated cells and in cells treated with C3 transferase (B) or the p160ROCK inhibitor Y-27632 (C). Integrin activation was induced as described in the legend of Figure 1B, and PDGF (25 ng/ml) and LPA (1 μM) were added 10 min before acid loading. Data are expressed relative to control (quiescent) recovery rates in untreated cells and represent the mean ± SEM of three to four separate cell preparations.