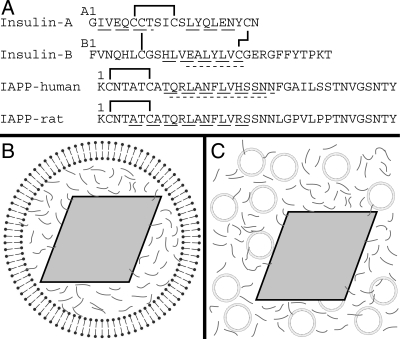
Figure 1.
Schematic of peptide sequences and experimental conditions. (A) Sequences of insulin A and B chains, as well as human and rat versions of IAPP. Solid lines indicate disulfide bonds. Wide dashed underlines indicate regions identified as α-helical either in the T6 crystal structure of insulin (1MSO) (Smith et al. 2003), by EPR for membrane-bound human IAPP (Apostolidou et al. 2008), or by NMR for rat IAPP in solution (Williamson and Miranker 2007). Regions that bind the catalytic cleft of IDE are underlined with short dashes (Shen et al. 2006). (B) Schematic of an insulin secretory granule. A dense crystalline granule core of insulin (gray rhombus) is surrounded by a lipid bilayer and by IAPP (gray lines) in the halo region. (C) Schematic of experimental conditions for insulin crystal binding experiments. Synthetic liposomes (circles) and IAPP are added to preparations of insulin crystals. Illustrations are not to scale.