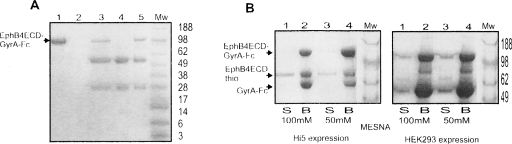
Figure 2.
Generation of Eph-ECD α-thioester. (A) Expression of the EphB4ECD-GyrA-Fc fusion protein. The protein was purified from the expression media by affinity chromatography using Protein-A Sepharose beads and resolved on SDS-PAGE as shown. (Mw) Molecular weight marker (in kilodaltons). (Lanes 1,3,5) Expression of the fusion protein in Hi5, Sf9, and HEK293 cells, respectively; (lanes 2,4) control (no transfection) Hi5 and Sf9 cells. The top band (MW ∼ 110 kDa) is the fusion protein. The two lower bands are impurities derived from the serum-rich cell growth media used in the HEK293 and Sf9 cell systems. (B) Generation of the EphB4 ECD α-thioester. The MESNA-induced cleavage of the EphB4ECD-GyrA-Fc fusion protein purified from Hi5 (left panel) and HEK293 (right) cells is resolved on SDS-PAGE. (Mw) Molecular weight marker (in kilodaltons). (Lanes 1,2) Cleavage with 100 mM MESNA; (lanes 3,4) cleavage with 50 mM MESNA. The uncleaved fusion protein and the cleaved intein-Fc fragment bind to the Protein-A Sepharose beads (B) as observed in lanes 2 and 4. The EphB4 ECD α-thioester fragment (EphB4ECDthio, MW ∼ 62 kDa) stays in the supernatant (S) as observed in lanes 1 and 3.