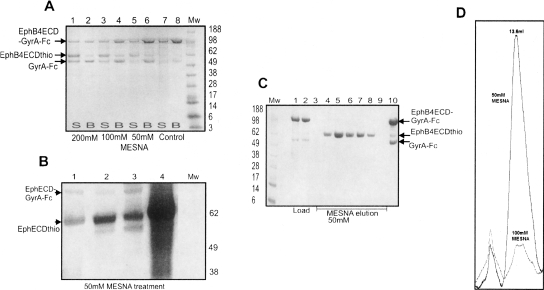
Figure 3.
Optimization of the Eph-ECD-thioester yield. (A) α-Thioester generation in the (2× Cys → Ser/Ser → Phe) EphB4ECD-GyrA-Fc mutant. SDS-PAGE after the treatment of fusion protein mutant with increasing concentration of MESNA as described in the text. (Mw) Molecular weight marker (in kilodaltons). (Lanes 7,8) Control with no MESNA added. The uncleaved fusion protein and the cleaved intein-Fc fragment bind to the Protein-A Sepharose beads (B) as shown in lanes 2,4, and 6. The amount of EphB4 ECD α-thioester fragment (EphB4ECDthio, MW ∼ 62kDa) generated in the supernatant (S) increases with increasing concentration of MESNA as shown in lanes 1,3, and 5. (B) Effect of mutations at the C terminus of Eph ECD on the thiol cleavage efficiency. SDS-PAGE showing the generation of the Eph ECD α-thioester from the different C-terminal mutants with 50 mM MESNA treatment. (Mw) Molecular weight marker (in kilodaltons). (Lanes 1–3) EphB4ECD α-thioester with glycine, phenylalanine, and alanine, respectively, at the C terminus. (Lane 4) EphA3 ECD α-thioester with phenylalanine at the C terminus. (C) Large-scale purification of EphB4 ECD with an active C-terminal α-thioester. MESNA induced on-column (Protein-A Sepharose column) cleavage of the mutated (2× Cys → Ser/Ser → Phe) EphB4ECD-GyrA-Fc fusion protein. (Mw) Molecular weight marker (in kilodaltons). (Lanes 1,2) Loaded fusion protein; (lanes 3–9) fractions containing the EphB4 ECD α-thioester eluted after 50 mM MESNA treatment; (lane 10) the uncleaved fusion protein and GyrA-Fc fragment remains bound to the Protein-A Sepharose column after cleavage. (D) Gel-filtration chromatography elution profile of the EphA3 ECD obtained after the cleavage of EphA3ECD-GyrA-Fc with 50 mM (solid line) and 100 mM (dotted line) MESNA. The protein is properly folded at the 50 mM thiol concentration, but becomes largely misfolded and aggregated as the concentration of MESNA is increased.