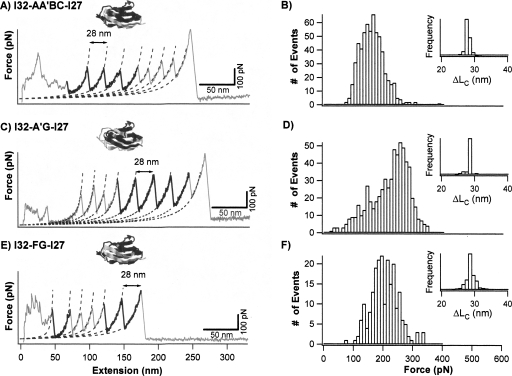
Figure 3.
Mechanical properties of mechanically stable hybrid Ig domains I32-AA′BC-I27, I32-A′G-I27, and I32-FG-I27. (A,C, and E) Typical force–extension curves of stretching heteropolyproteins (GB1-I32-AA′BC-I27)4, (GB1-I32-A′G-I27)4, and (GB1-I32-FG-I27)4, respectively. The unfolding events of GB1 are characterized by ΔLc of ∼18 nm and unfolding force of ∼180 pN, and are colored in gray. The unfolding events with ΔLc of ∼28 nm correspond to the unfolding of well-folded engineered hybrid Ig domains and are in black. Dotted lines correspond to WLC fits. (B,D, and F) Unfolding force histograms for hybrid Ig domains I32-AA′BC-I27, I32-A′G-I27, and I32-FG-I27, respectively. The unfolding forces of I32-AA′BC-I27 and I32-FG-I27 show unimodal distribution with average unfolding forces of 152 ± 39 pN (n = 602) and 198 ± 44 pN (n = 215), respectively. The unfolding forces of I32-A′G-I27 show a bimodal distribution, with the first peak centered at 150 pN and the second peak centered around 250 pN, respectively. Insets are histograms for ΔLc of individual hybrid Ig domains.