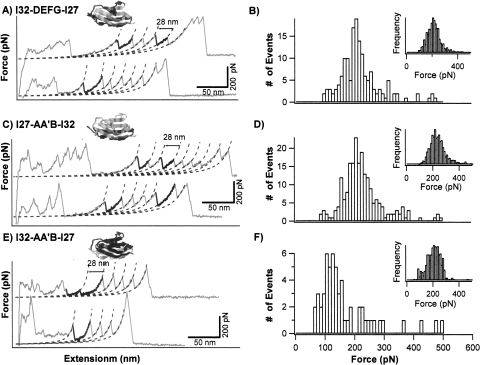
Figure 5.
Hybrid Ig domains I32-DEFG-I27, I27-AA′B-I32, and I32-AA′B-I27 exhibit duality in their mechanical stability. (A,C, and E) Typical force–extension curves of (GB1-I32-DEFG-I27)4, (GB1-I27-AA′B-I32)4, and (GB1-I32-AA′B-I27)4, respectively. The unfolding events of GB1 are colored in gray. The unfolding events with ΔLc of ∼28 nm correspond to the unfolding of hybrid Ig domains and are in black. It is of note that the number of GB1 unfolding events in the force–extension curves are much greater than the number of the unfolding events of hybrid Ig domains. (B,D, and F) Unfolding force histograms of I32-DEFG, I27-AA′B-I32, and I32-AA′B-I27 domains in the heteropolyproteins. The number of events in these histograms is 154, 211, and 57, respectively. Insets in B, D, and F are unfolding force histograms of GB1 domains in the heteropolyproteins, respectively. The number of events is 570, 1199, and 377, respectively.