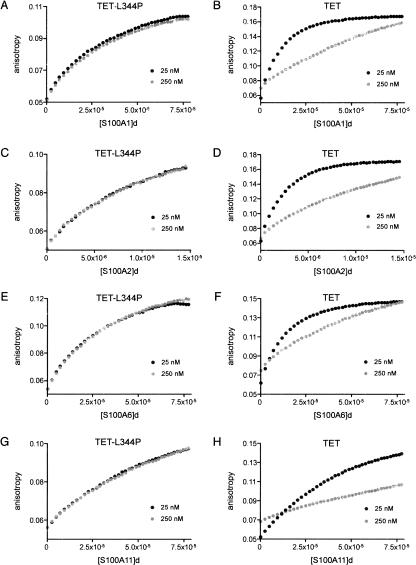
Figure 2.
Binding of S100 proteins to p53 TET and TET-L344P peptides. Binding of S100A1 (A,B), S100A2 (C,D), S100A6 (E,F), and S100A11 (G,H) to fluorescein-labeled TET-L344P (A,C,E,G) and TET (B,D,F,H) peptides by using fluorescence anisotropy is shown. The data shown are the mean of three independent measurements. Peptides were studied at two different concentrations, 25 nM and 250 nM. The 250 nM sample was prepared as a 9:1 mixture of nonlabeled:labeled peptide to normalize the fluorescence intensity to the 25 nM sample and to avoid energy transfer effects. The S100 protein dimer concentration is expressed in molar units (M). The S100 stock was 400 μM dimer, except in experiments with S100A2 (75 μM), which shows tight affinity for TET peptide.