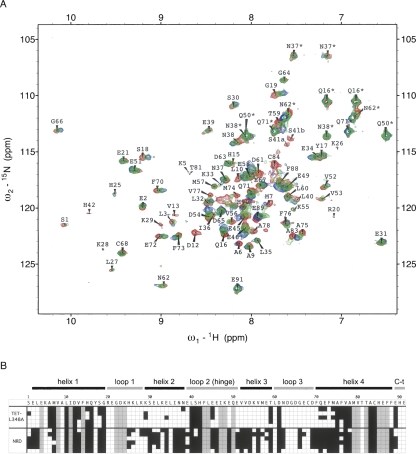
Figure 5.
Perturbation of 15N-S100B chemical shifts upon binding to NRD and TET-L348A. (A) 500 MHz 2D fast-HSQC of 80 μM 15N-S100B alone (red), or in the presence of 100 μM TET-L348A (green) or 100 μM unlabeled NRD (blue). Assignments with an asterisk denote peaks for side-chain amides. (B) The amino acid sequence of S100B is indicated with a one-letter code, with elements of secondary structure shown above. 15N-S100B (80 μM) was mixed with either 80–100 μM TET-L348A or 100–300 μM NRD. Shading in the squares indicates residues for which the N-H correlation for S100B is perturbed by >0.04 ppm (black), unperturbed (white), or unavailable assignments (gray). Each line is an independent experiment with each peptide (five for TET-L348A and six for NRD).