Fig. 4.
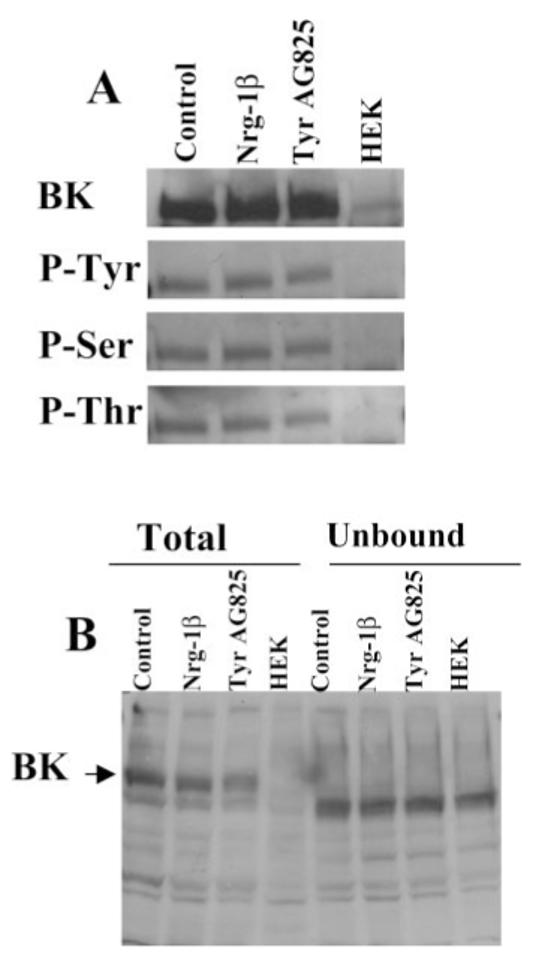
Tyrosine, serine, and threonine phosphorylation status of BK channels is unchanged in Nrg-1β- or TyrAG825-treated cells. D54 cells were treated overnight with vehicle, Nrg-1β, or TyrAG825, and cells were lysed and protein harvested. A: Cell lysates were immunoprecipitated with an anti-BK antibody (Chemicon) and then probed with an anti-BK (top), antiphosphotyrosine (top, middle), antiphosphothreonine (bottom, middle), and antiphosphothreonine (bottom). Each band in this figure had an approximate molecular weight of 120 kD. B: Equal amounts of protein from whole-cell lysates and the unbound fractions were analyzed by Western blot analysis to demonstrate a complete immunoprecipitation of the BK channel from our samples. Note that both antibodies used in this study consistently recognized a band slightly lower than the band at 120 kD (BK); this band was always present in the HEK cell lanes, and we presume it to be nonspecific binding.
