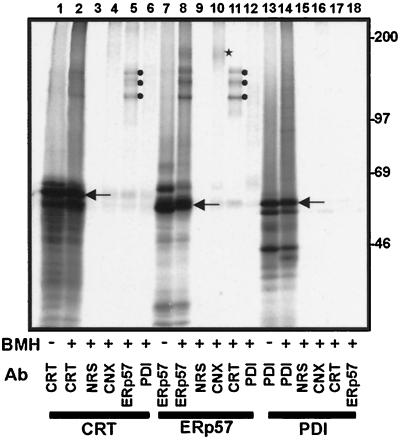
Figure 3.
Interactions of in vitro synthesized calreticulin, ERp57, and PDI with the endogenous ER proteins of microsomes. Calreticulin (CRT, lanes 1–6), ERp57 (lanes 7–12), and PDI (lanes 13–18) RNAs were translated in a rabbit reticulocyte lysate system in the presence of microsomes. After termination of translation, the microsomal fraction was isolated and, where indicated, the samples were treated with the cross-linking reagent BMH. The reaction was quenched, and the samples denatured with 1% SDS before immunoprecipitation with the antisera indicated: CRT, anticalreticulin; NRS, control nonrelated serum; CNX, anti-calnexin; ERp57, anti-ERp57; PDI, anti-PDI. The samples were analyzed on an 8% SDS-polyacrylamide gel. The identity of imported polypeptides with cleaved signal sequences (indicated by arrows) was confirmed by protease protection and comparison with unprocessed polypeptides bearing signal sequences (our unpublished observations).