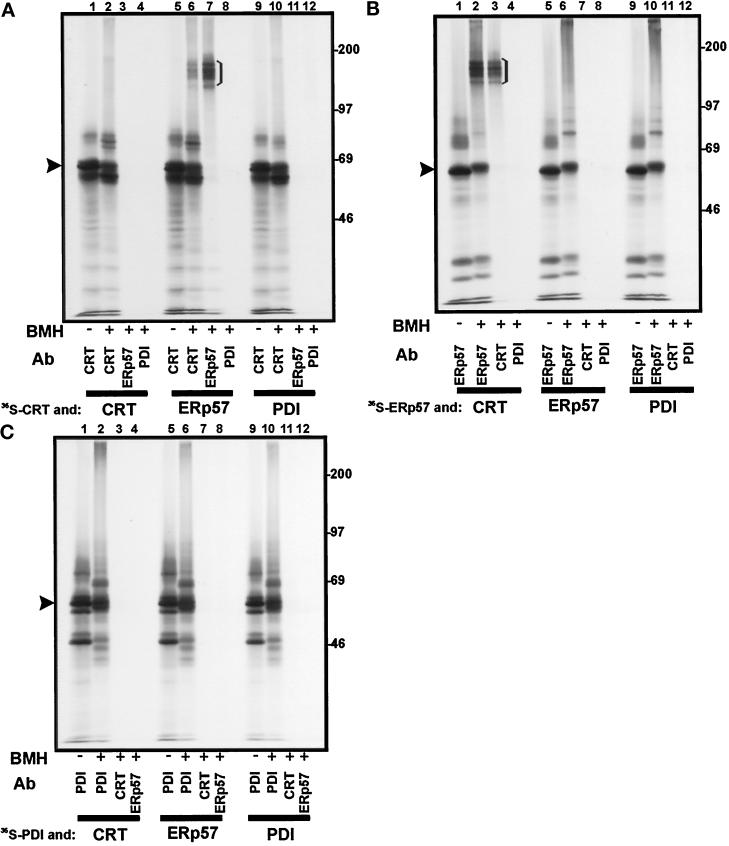
Figure 5.
Interactions between in vitro translated calreticulin, ERp57, and PDI in solution. The products of a translation reaction carried out in the presence of [35S]methionine were mixed with the products of a translation reaction carried out with unlabeled methionine, and the resulting protein complexes were cross-linked with BMH. 35S-labeled calreticulin (panel A), ERp57 (panel B), and PDI (panel C) were mixed with unlabeled calreticulin (CRT, lanes 1–4), ERp57 (lanes 5–8), and PDI (lanes 9–12). After cross-linking and SDS denaturation, immunoprecipitation was carried out with the antisera indicated (see Figure 3 for key). The samples were analyzed on an 8% SDS-polyacrylamide gel. The identity of full-length precursor proteins (i.e., with signal sequences; indicated by arrowheads) was experimentally confirmed (our unpublished observations).