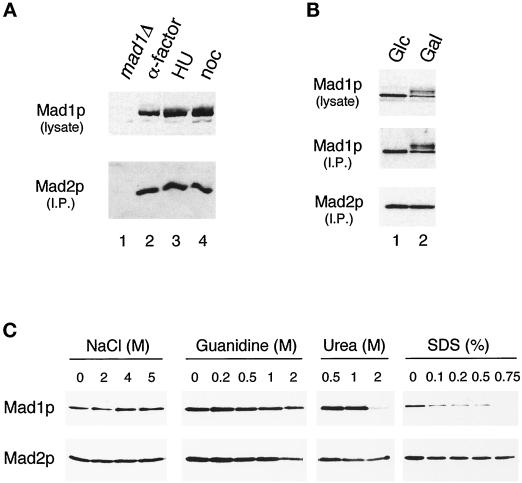
Figure 4.
Regulation of the Mad1–Mad2p complex. (A) The Mad1p–Mad2p complex is similar in cells arrested at G1, S, and M phases. Strains were grown to log phase and then arrested for 3 h in G1 (with α-factor; lane 2), in S phase (with hydroxyurea; lane 3), or in mitosis (with nocodazole; lane 4) before harvesting. Mad1p was immunoprecipitated from the extracts and then immunoblotted with an anti-Mad2p antibody (lower panel). The upper panel is an immunoblot of the Mad1p present in the lysates. In lane 1 a mad1Δ strain is used as a control; this strain was also treated with nocodazole. (B) Mad1p–Mad2p complex formation is independent of the phosphorylation state of Mad1p. All species of Mad1p co-immunoprecipitate with Mad2p in cells overexpressing Mps1p. Mad2p was immunoprecipitated and immunoblotted with an anti-Mad1p (upper panel) or an anti-Mad2p (lower panel) antibody. Lane 1, cells containing MPS1 under the control of galactose-inducible promoter were repressed for Mps1 expression by culturing in media containing glucose (Glc); lane 2, the same strain of cells was grown in galactose (Gal) to induce Mps1p overexpression. (C) Mad1p and Mad2p form a tight complex. Extracts from cells expressing hexahistidine-tagged Mad2p were applied to nickel-nitrilotriacetic acid beads. The gels show the proteins that remain on the beads after washing with the indicated concentrations of sodium chloride, guanidine hydrochloride, urea, or SDS. Samples were immunoblotted with an anti-Mad1p (upper panel) or an anti-Mad2p (lower panel) antibody.