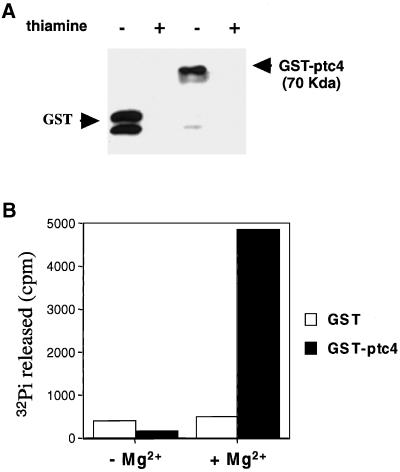
Figure 3.
Casein phosphatase activity of Ptc4 protein produced in S. pombe. Using GSH-Sepharose beads, GST and GST-Ptc4 fusion proteins were purified from total lysates of wild-type (PR109) cells transformed with pREP1-GST or pREP1-GST-ptc4+. Cells were grown for 15 h in thiamine-depleted medium before harvest. (A) Purification of the GST and GST-Ptc4 was confirmed by SDS-PAGE and immunoblotting using anti-GST antibody. (B) GST and GST-Ptc4 were incubated with 32P-labeled casein in the presence or absence of 20 mM MgCl2. Activity is shown as the amount of 32Pi (cpm) released in the reaction mixture.