Abstract
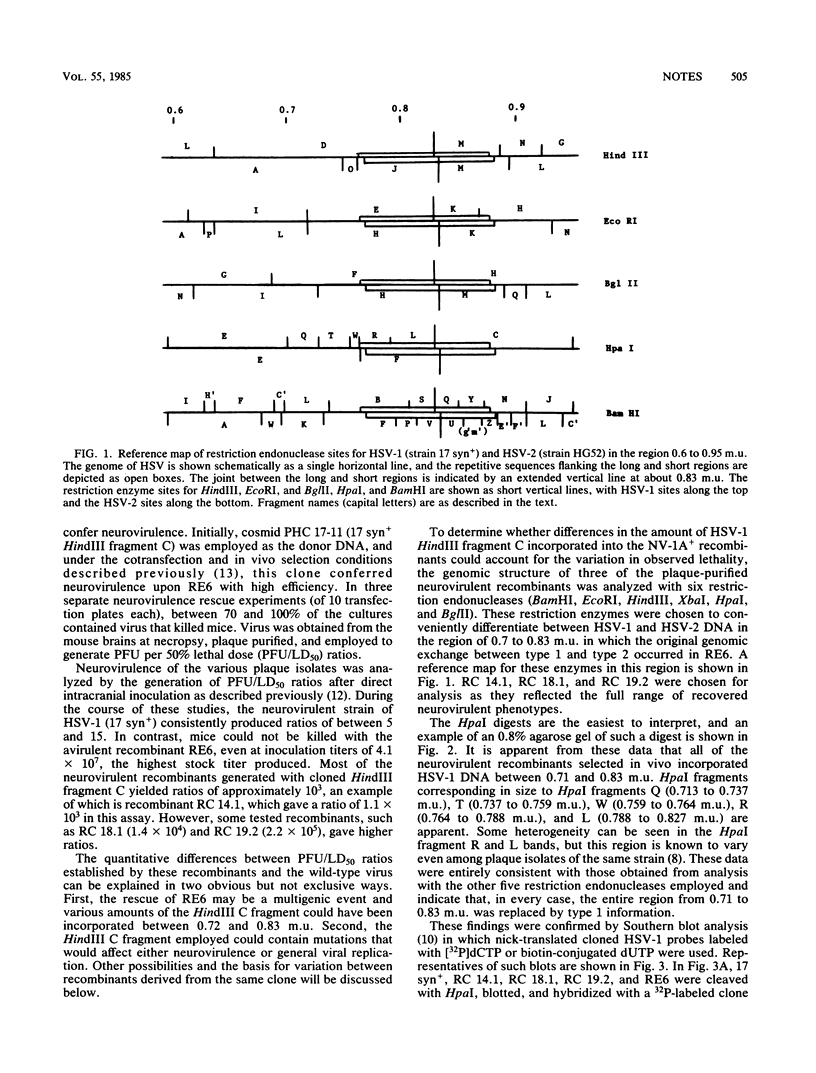
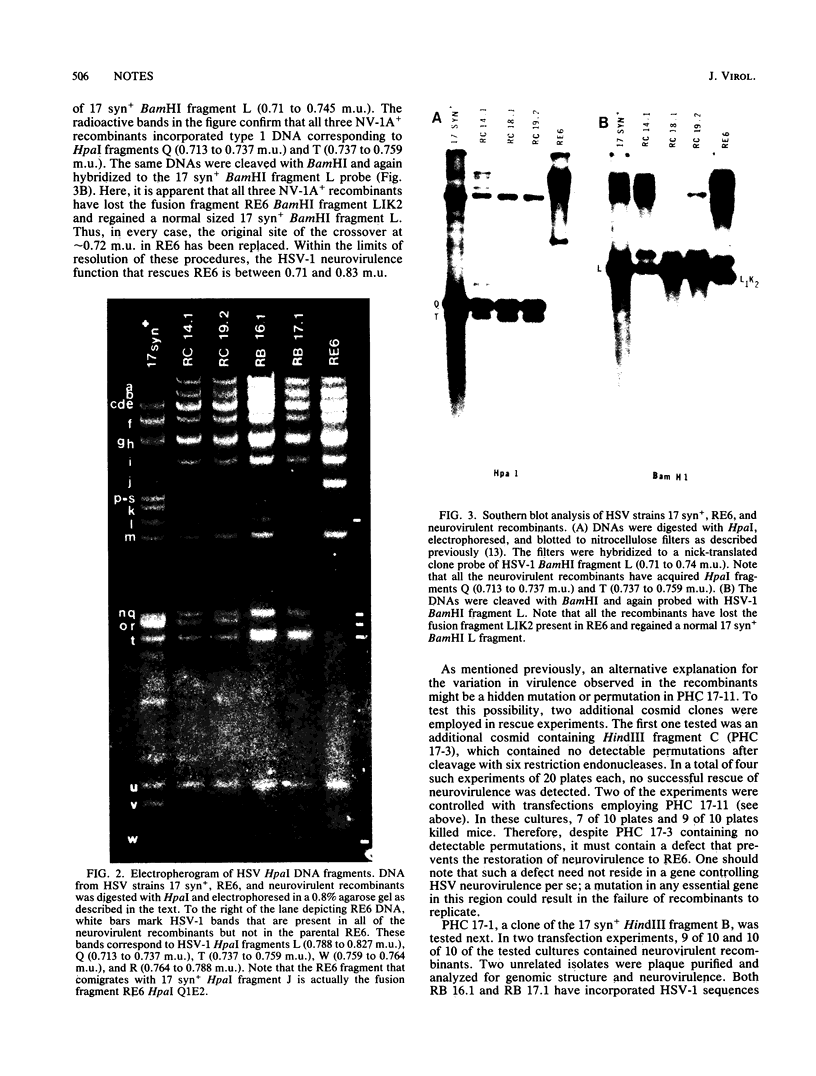
A herpes simplex virus type 1 (HSV-1) genetic function that is required for viral replication in the murine central nervous system was unambiguously localized. Thus, cosmid clones of either HSV-1 HindIII fragment C (0.64 to 0.87 map units) or fragment B (0.64 to 0.83 plus 0.91 to 1.0 map units) were employed to restore neurovirulence to an intertypic recombinant (RE6) that is specifically deficient in this property. The neurovirulent recombinants were generated in cell culture by cotransfecting the clone fragments and unit-length RE6 DNA and then selected in mouse brains. Either fragment efficiently conferred neurovirulence to RE6, demonstrating that no short region unique sequences are required. Analyses of the genomic structures of the neurovirulent recombinants showed that, in every case, HSV-1 information from 0.71 to 0.83 map units was incorporated into the RE6 genome. Cleavage of HindIII fragment C with EcoRI eliminated its capacity to rescue RE6. Virulence could be restored by the addition of HSV-1 BamHI fragment L (0.71 to 0.74 map units) that spans an EcoRI site at 0.72 map units. The precise location of this HSV-1 neurovirulence function is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Centifanto-Fitzgerald Y. M., Yamaguchi T., Kaufman H. E., Tognon M., Roizman B. Ocular disease pattern induced by herpes simplex virus is genetically determined by a specific region of viral DNA. J Exp Med. 1982 Feb 1;155(2):475–489. doi: 10.1084/jem.155.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix R. D., McKendall R. R., Baringer J. R. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun. 1983 Apr;40(1):103–112. doi: 10.1128/iai.40.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaerner H. C., Schröder C. H., Ott-Hartmann A., Kümel G., Kirchner H. Genetic variability of herpes simplex virus: development of a pathogenic variant during passaging of a nonpathogenic herpes simplex virus type 1 virus strain in mouse brain. J Virol. 1983 Apr;46(1):83–93. doi: 10.1128/jvi.46.1.83-93.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knotts F. B., Cook M. L., Stevens J. G. Latent herpes simplex virus in the central nervous system of rabbits and mice. J Exp Med. 1973 Sep 1;138(3):740–744. doi: 10.1084/jem.138.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Stevens J. G. Biological characterization of a herpes simplex virus intertypic recombinant which is completely and specifically non-neurovirulent. Virology. 1983 Nov;131(1):171–179. doi: 10.1016/0042-6822(83)90543-3. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Wagner E. K., Stevens J. G. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology. 1983 Nov;131(1):180–192. doi: 10.1016/0042-6822(83)90544-5. [DOI] [PubMed] [Google Scholar]