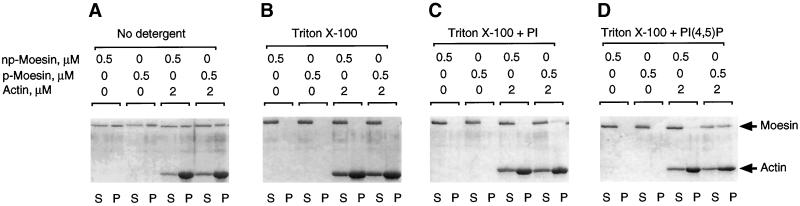
Figure 7.
Phosphatidylinositol 4,5-biphosphate is required for F-actin binding of phosphorylated moesin in Triton X-100. Phosphorylated (p-) and non-phosphorylated (np-) moesin isolated from platelets was incubated alone or in the presence of α-actin for 1 h at 37°C before sedimentation. In A, no detergent was added, and sizable fractions (∼60%) of p- and np-moesin sedimented and were recovered in the pellet together with actin. There was no apparent difference between the two forms of moesin. In B, 0.1% Triton X-100 was added to the reaction mixtures. Both forms of moesin were soluble and did not sediment under this condition. In C and D, mixed micelles of 0.1% Triton X-100 and 0.01% phosphatidylinositol (PI) or phosphatidylinositol 4,5-biphosphate (PIP2) were added to the reaction mixtures. Approximately 50% of the total p-moesin co-sedimented together with actin in the presence of PIP2. In all experiments, equal volumes of supernatant (S) and pellet (P) fractions were analysed by SDS-PAGE and Coomassie blue staining.