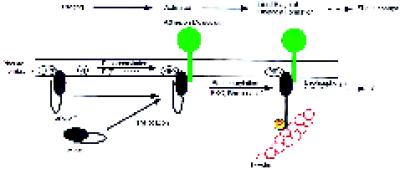
Figure 9.
A multistep model for the regulation of the F-actin-binding activity of moesin in platelets. In the resting state, moesin is bound to as-yet-unknown membrane proteins or sites indicated by phosphatidylinositol polyphosphate (PIPx). Membrane-bound moesin may exist in equilibrium with a soluble, cytosolic form. A first signal, perhaps involving PI(P) kinases, elevates levels of PIP2. Moesin in a complex with other membrane components undergoes a conformational change, allowing it to become a substrate for a membrane-associated kinase, such as PKC or Rho-kinase. After phosphorylation at 558Thr, the F-actin binding function of moesin is activated, and linkage with actin filaments in the vicinity of the membrane may occur. Steady-state phosphorylation is maintained by the action of protein kinase(s) and protein phosphatase(s). Staurosporine and calyculin A shift the balance toward complete dephosphorylation or phosphorylation, respectively. Similarly, transient changes in the number of phosphorylated and activated moesin molecules, caused by an increase in kinase or a decrease in phosphatase activity, enhance the potential to form new membrane–cytoskeletal links.