Abstract
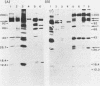
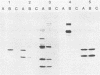
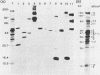
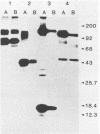
Herpesvirus saimiri is a lymphotrophic virus isolated from squirrel monkeys that causes highly malignant lymphomas in owl monkeys, marmosets, and rabbits, but not in its natural host. We have been interested in exploring immunological and biological aspects of this phenomenon and describe in this report the isolation of 27 monoclonal antibodies (MCAs) to herpesvirus saimiri which were grouped into 11 distinct sets based on the proteins they immunoprecipitated. In total, these 11 groups of MCAs identify the majority of the proteins present in the virion. Immunofluorescence was used to study how the viral antigens are compartmentalized in infected cells. One antibody produced intense nuclear staining and immunoprecipitated both the largest protein seen in gels (150,000 daltons) and one of the smallest (13,000 daltons). All of the other groups of MCAs principally stained the cytoplasm of infected cells. One of the unexpected results of this study was the observation that a majority of the MCAs precipitated more than one protein (15 of 27 antibodies, 7 of 11 groups). Whereas one group of MCAs, which normally precipitated four proteins, identified only single polypeptides after dithiothreitol pretreatment, the other sets of antibodies continued to recognize two or more viral antigens under reducing conditions. Immunoprecipitation of viral proteins with polyvalent sera obtained from virus-infected rabbits and monkeys was carried out to verify and extend previously published reports on the proteins of herpesvirus saimiri.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daniel M. D., Meléndez L. V., Hunt R. D., King N. W., Anver M., Fraser C. E., Barahona H., Baggs R. B. Herpesvirus saimiri: VII. Induction of malignant lymphoma in New Zealand white rabbits. J Natl Cancer Inst. 1974 Dec;53(6):1803–1807. [PubMed] [Google Scholar]
- Desrosiers R. C., Falk L. A. Herpesvirus saimiri strain variability. J Virol. 1982 Jul;43(1):352–356. doi: 10.1128/jvi.43.1.352-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. Herpesvirus saimiri DNA in tumor cells--deleted sequences and sequence rearrangements. J Virol. 1981 Aug;39(2):497–509. doi: 10.1128/jvi.39.2.497-509.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk L. A., Nigida S. M., Deinhardt F., Wolfe L. G., Cooper R. W., Hernandez-Camacho J. I. Herpesvirus ateles: properties of an oncogenic herpesvirus isolated from circulating lymphocytes of spider monkeys (Ateles sp.). Int J Cancer. 1974 Oct 15;14(4):473–482. doi: 10.1002/ijc.2910140407. [DOI] [PubMed] [Google Scholar]
- Falk L., Johnson D., Deinhardt F. Transformation of marmoset lymphocytes in vitro with Herpesvirus ateles. Int J Cancer. 1978 May 15;21(5):652–657. doi: 10.1002/ijc.2910210517. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Mulder C., Werner F. J., Daniel M. D., Falk L. A., Delius H. Herpesvirus ateles DNA and its homology with Herpesvirus saimiri nucleic acid. J Virol. 1978 Jan;25(1):361–373. doi: 10.1128/jvi.25.1.361-373.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W. Structure and function of herpesvirus saimira DNA. IARC Sci Publ. 1975;(11 Pt 1):145–149. [PubMed] [Google Scholar]
- Hunt R. D., Garcia F. G., Barahona H. H., King N. W., Fraser C. E., Meléndez L. V. Spontaneous Herpesvirus saimiri lymphoma in an owl monkey. J Infect Dis. 1973 Jun;127(6):723–725. doi: 10.1093/infdis/127.6.723. [DOI] [PubMed] [Google Scholar]
- Johnson D. R., Jondal M. Herpesvirus ateles and herpesvirus saimiri transform marmoset T cells into continuously proliferating cell lines that can mediate natural killer cell-like cytotoxicity. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6391–6395. doi: 10.1073/pnas.78.10.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G., Fleckenstein B., Bodemer W. Structural proteins of Herpesvirus saimiri. J Virol. 1983 Sep;47(3):463–470. doi: 10.1128/jvi.47.3.463-470.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Pearson G., Rabson A., Ablashi D. V., Falk L., Wolfe L., Dienhardt F., Rabin H. Antibody reactions to herpesvirus saimiri (HVS)-induced early and late antigens (EA and LA) in HVS-infected squirrel, marmoset and owl monkeys. Int J Cancer. 1973 Jul 15;12(1):270–289. doi: 10.1002/ijc.2910120128. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leinbach S. S., Reno J. M., Lee L. F., Isbell A. F., Boezi J. A. Mechanism of phosphonoacetate inhibition of herpesvirus-induced DNA polymerase. Biochemistry. 1976 Jan 27;15(2):426–430. doi: 10.1021/bi00647a029. [DOI] [PubMed] [Google Scholar]
- Melendez L. V., Daniel M. D., Hunt R. D., Garcia F. G. An apparently new herpesvirus from primary kidney cultures of the squirrel monkey (Saimiri sciureus). Lab Anim Care. 1968 Jun;18(3):374–381. [PubMed] [Google Scholar]
- Melendez L. V., Hunt R. D., King N. W., Barahona H. H., Daniel M. D., Fraser C. E., Garcia F. G. Herpesvirus ateles, a new lymphoma virus of monkeys. Nat New Biol. 1972 Feb 9;235(58):182–184. doi: 10.1038/newbio235182b0. [DOI] [PubMed] [Google Scholar]
- Melèndez L. V., Hunt R. D., Daniel M. D., Fraser C. E., Barahona H. H., King N. W., Garcìa F. G. Herpesviruses saimiri and ateles--their role in malignant lymphomas of monkeys. Fed Proc. 1972 Nov-Dec;31(6):1643–1650. [PubMed] [Google Scholar]
- Meléndez L. V., Hunt R. D., Daniel M. D., García F. G., Fraser C. E. Herpesvirus saimiri. II. Experimentally induced malignant lymphoma in primates. Lab Anim Care. 1969 Jun;19(3):378–386. [PubMed] [Google Scholar]
- Nowinski R. C., Lostrom M. E., Tam M. R., Stone M. R., Burnette W. N. The isolation of hybrid cell lines producing monoclonal antibodies against the p15(E) protein of ecotropic murine leukemia viruses. Virology. 1979 Feb;93(1):111–126. doi: 10.1016/0042-6822(79)90280-0. [DOI] [PubMed] [Google Scholar]
- O'Hare P., Honess R. W. Identification of a subset of herpesvirus saimiri polypeptides synthesized in the absence of virus DNA replication. J Virol. 1983 Apr;46(1):279–283. doi: 10.1128/jvi.46.1.279-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Luka J., Klein G., Daniel M. D. Detection of a nuclear antigen in Herpesvirus ateles-carrying marmoset lines by the acid-fixed nuclear binding technique. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2042–2046. doi: 10.1073/pnas.76.4.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall R. E., Honess R. W., O'Hare P. Proteins specified by herpesvirus saimiri: identification and properties of virus-specific polypeptides in productively infected cells. J Gen Virol. 1983 Jan;64(Pt 1):19–35. doi: 10.1099/0022-1317-64-1-19. [DOI] [PubMed] [Google Scholar]
- Randall R. E., Honess R. W. Proteins specified by Herpesvirus saimiri: purification and properties of a single polypeptide which elicits virus-neutralizing antibody. J Gen Virol. 1982 Jan;58(Pt 1):149–161. doi: 10.1099/0022-1317-58-1-149. [DOI] [PubMed] [Google Scholar]
- Schirm S., Müller I., Desrosiers R. C., Fleckenstein B. Herpesvirus saimiri DNA in a lymphoid cell line established by in vitro transformation. J Virol. 1984 Mar;49(3):938–946. doi: 10.1128/jvi.49.3.938-946.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]