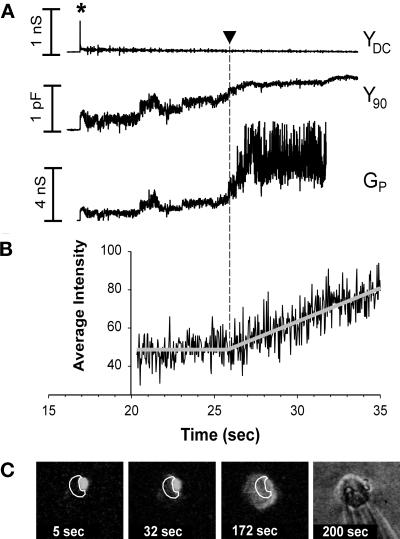
Figure 7.
Simultaneous electrical recording of fusion pores and video fluorescence measurements of R18 transfer for RBC fusion to WT HA-expressing COS7 cells. (A) The pH of the solution was lowered at 0 s. The opening of a fusion pore between a WT HA-expressing cell and an RBC was marked by a spike in YDC (at ∼17 s) and a simultaneous increment in Y90. YDC returned nearly to baseline after the spike, showing that both the COS7 and RBC membrane conductances remained small. The fusion pore conductance (Gp, bottom trace) was calculated from the Y90 trace (see MATERIALS AND METHODS). With enlargement of the fusion pore, the voltage applied across the COS7 cell membrane increasingly penetrated into the RBC, and Y90 was observed to increase. The voltage fully penetrated into the RBC once the pore enlarged sufficiently, and hence the precise value of Gp (Figure 7A) became less certain, calculated as a “noisy” Gp, once the Y90 neared saturation. (B) The time-dependent fluorescence intensities shown in C were fit with two straight lines, a constant fluorescence followed by a linearly increasing fluorescence. Their intersection determined the lag time from acidification until the onset of R18 spread (here 25.8 s, shown by arrowhead in A and B). (C) To temporally correlate the onset of membrane dye redistribution with conductance increases, for each experiment an ROI was selected for a COS7 cell (white contours), and the average intensity of fluorescence of this region, adjacent to a bound RBC, was determined on a frame-by-frame basis. The fluorescence images shown were obtained by averaging eight sequential video frames. The four images represent (from left to right) fluorescence patterns before dye spread, soon after dye spread began, after complete redistribution of R18, and the bright-field image at the conclusion of the experiment. Times after reducing the pH are indicated on all images and graphs.