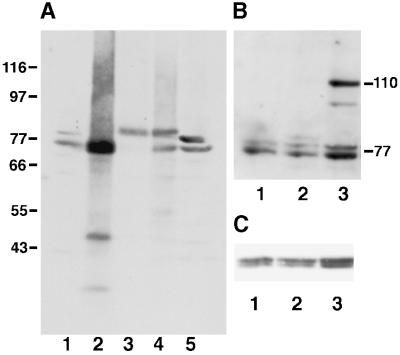
Figure 1.
Characterization of HN184 antibody. Protein extracts from different S. pombe strains were analyzed by immunoblots and probed with HN184 antisera (A, B) or the anti-α tubulin antibody TAT1 as a loading control (C). (A) A wild-type cell (IH365) extract shows a Plo1 doublet of the expected size of ∼77 kDa (lane 1). The intensity of the lower band increased in wild-type cells, which expressed plo1+ from a multicopy plasmid under the control of the nmt41 promoter (IH1409) (lane 2). In a strain from which the genomic copy of plo1+ has been deleted, and the cells were kept alive by the expression of an HA-tagged plo1+ gene from the multicopy plasmid p41plo1.NHA, the two wild-type bands were replaced by a single band at 80 kDa (IH1391) (lane 3). As predicted from lanes 1–3, two bands were seen in a wild-type strain overexpressing the HA-tagged protein (IH1289), a bottom wild-type band and an upper HA-tagged band (lane 4). The intensity of the upper band in wild-type cells was increased in extracts prepared in the presence of 0.5 μM okadaic acid (lane 5). Blotting with TAT1 showed similar loading levels in each lane. (B) Lane 1, IH365 extract. Lane 2, extract of IH1313 showing the faint additional HA-tagged plo1 band at 80 kDa. Lane 3, extract of IH1314 showing an additional GFP- and HA-tagged band of Plo1 at 110 kDa. C shows the same filter as depicted in B, probed with TAT1 to demonstrate consistency of loading.