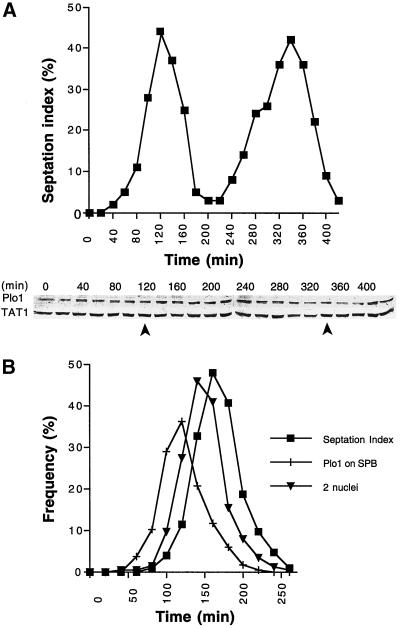
Figure 3.
Fluctuations in Plo1 protein levels do not account for the cell cycle specificity of Plo1 localization to the SPB. (A) A cell cycle-synchronized population of wild-type cells (IH365) was generated by elutrient centrifugation at 25°C in appropriately supplemented EMM2. The small G2 cells were harvested at the beginning of the experiment, and samples were then taken every 20 min for Western blot analysis and scoring the septation index (n = 200 for each time point). The top panel shows the septation index, which indicates good cell cycle synchrony in the culture, and the bottom panel shows a Western blot of whole-cell extracts. The blot was cut into two, and the top portion was probed with antibodies to Plo1 and the bottom portion was probed with antibodies to a tubulin (TAT1). The tubulin blot acts as a loading control. The position of the peaks seen in the septation index are indicated by arrows beneath the blots. Quantitation of the ratio of the TAT1 to Plo1 signals for two independent blots for each of two independent experiments (each of which had two complete cell cycles) led to the conclusion that there is no apparent fluctuation in Plo1 levels as cells progress through the cell cycle. (B) Anti-Plo1 immunofluorescence analysis of an elutrient centrifugation synchronized population of wild-type cells growing in EMM2 at 25°C. Small G2 cells were harvested at the beginning of the time course at time point 0 and processed every 20 min for anti-Plo1 immunofluorescence microscopy. The septation index of each sample was determined by calcofluor staining before the cells were processed for immunofluorescence microscopy. Plo1 association with the SPB precedes anaphase B by ∼20 min and septation by ∼30 min. n = 200 for each data point. A repeat of this experiment gave similar profiles.