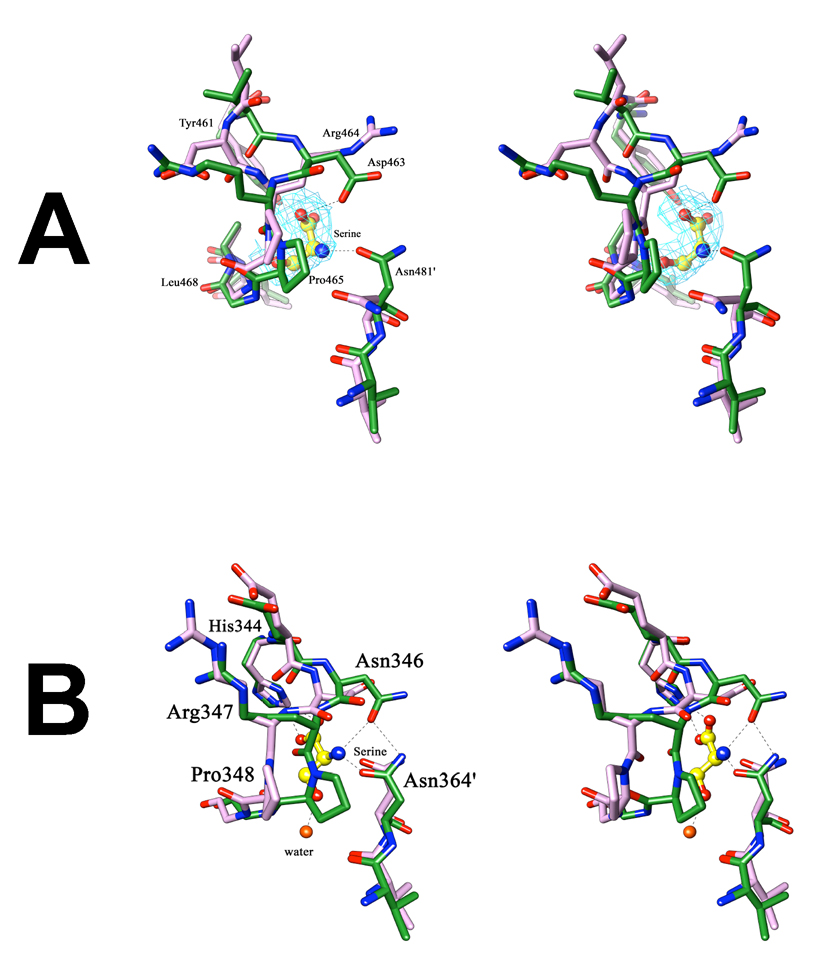
Figure 9. Stereoview of the changes occurring at the allosteric site upon serine binding.
(A) In M.tb PGDH, superimposition of the regulatory domain of chain A of the apo-enzyme (purple) and the serine bound form (green) shows a flip of the side chains of Asp463 and Arg464, present on the loop region lining the allosteric site. Also seen is the change in orientation of Asn481′ from the adjacent subunit, which covers the allosteric site upon serine binding. Serine is depicted in yellow ball and stick. The Shake &wARP unbiased 2Fo−Fc electron density map of serine is shown in light blue (1σ level contour). (B) In E.coli PGDH, superimposition of the regulatory domain of chain A of the apo-form (purple) with the serine inhibited form (green) shows a transition of the loop region at Pro348, without side-chain flipping. In this case, transition of the Pro348 covers the allosteric site upon serine binding. Serine is shown at this site in the form of ball and stick (yellow). In both figures, the hydrogen bond interactions of serine with the protein are shown as dashed lines, oxygen atoms are shown in red, and nitrogen atoms in blue.